INTRODUCTION
Ussurian bullhead Leiocassis ussuriensis is fish that belongs to family Bagridae and is found in Daedong river, Amnok river, Imjin river, Han river, and Geum river. It has recently been found in nakdong river as well (Kim, 1997). They are nocturnal fish that live in rivers with slow current and in river bottom composed of mud or sand. With their chewy flesh and excellent taste of umami, they are essential in cooking fresh water spicy fish stew, along with the Korean bullhead.
As the reduction in available habitat due to recent environment pollution and development of rivers and flood have accelerated the decline of resources, it is necessary to work on resource recovery and increase in production in a timely manner. The most important and fundamental mission to do for the restoration of ussurian bullhead is to develop seedling production techniques by obtaining fertile eggs through artificial maturation and ovulation. To induce maturation and ovulation in fish species that does not naturally give birth indoors, hormones are artificially injected. And it is of great importance to inject the right type of hormone using the right method depending on the target species. Hormones that are currently being used in order to induce fish maturation and ovulation are pituitary extracts from fish and frogs, human chorionic gonadotropin (hCG), gonadotropin (GTH) of fish, luteinizing hormone releasing hormone (LHRH) of mammals, luteinising hormone releasing hormone analogue (LHRHa) that is known to be 30 to 50 times more effective than natural LHRH, and pimozide that is cheap and accessible (Park et al., 1998). These hormones in combination with pimozide has recently been used in bullhead (Silurus asotus, kwon et al., 1996), river puffer (Takifugu obscurus, Jang, 1996), and ussurian bullhead (Lim et al., 2012).
There have been studies on the species used in present study as a subject to the culture in the high value-added industry, such as the ultrastructure of spermatozoa of the ussurlan bullhead and phylogenetic discussion (Kim & Lee, 2000), the early gonadogenesis and sex differentiation in the bagrid catfish (Park et al., 2001), and the difference in the growth between male and female of ussurian bullhead (Kang et al., 2003), but not on the artificial egg collection techniques. Since it is hard to induce natural births in Ussurian bullhead, the ovulation inducement rate, ovulation time, fertilization rate, and hatching rates at differing concentrations of hormones were investigated in this study to examine the effectiveness of LHRHa, hCG, ovaprim, and pimozide in ovulation inducement in order to develop artificial egg collection techniques and to use these data as the foundation of development of the seedling production of ussurian bullhead.
MATERIALS AND METHODS
820 Fish (485 females and 335 males) born from natural mother between June 30th, 2005 and August 6th, 2008, were used and their weights are recorded in Table 1. Individuals with finished yolk accumulation were used. Once polyethylene cannula (2.5 mm inner diameter) was injected into the ovarian cavity of ussurian bullhead with expanded abdominal, their oogonia were collected by suction, and they were then put in 10% formaldehyde solution (Duksan Pure Chemicals Co. LTD) in order for their maturity to be examined under the microscope.
In order to examine the influence of injections of hormones on fish, the concentrations of hCG and LHRHa used were 500, 10,000, 20,000, and 30,000 IU/kg and 50, 100, 200, 300, and 400 μg/kg, respectively, and 0.75% NaCl was used as control. The concentrations of ovaprim were 1.0, 2.0, 2.5, and 3.5 ml/kg and thecombinations of LHRHa and pimozide were obtained by mixing 50, 100, 200, 300, and 400 μg/kg of LHRHa with 1,000 μg/kg of pimozide. Ovulation rate, ovulation time, fertilization rate, and hatching rate were examined.
hCG (Daesung Microbiological Labs. Co., LTD) was kept at –20°C by dilution until the experiment. LHRHa (Sigma Co., USA), ovaprim (Sigma Co., USA) and pimozide (Sigma Co., USA) were dissolved with 0.75% NaCl and then were kept at –20°C. The number of fish treated with each hormone was 76, 92, 80, and 69, respectively. Different concentrations were first injected to dorsal muscles and then accommodated in the water bath for ovulation inducement. The dry method was used for fertilization of eggs obtained by abdominal pressure and sperms and hatching temperature was kept at 26 +/–1°C. Fertilization and hatching rates were calculated by averaging those of randomly selected fertilized eggs.
Ovulation time was measured by lightly pressing on the abdominal region every hour and seeing if the eggs had been released, and only the individuals that released eggs smoothly were considered to be successes of ovulation inducement.
RESULTS
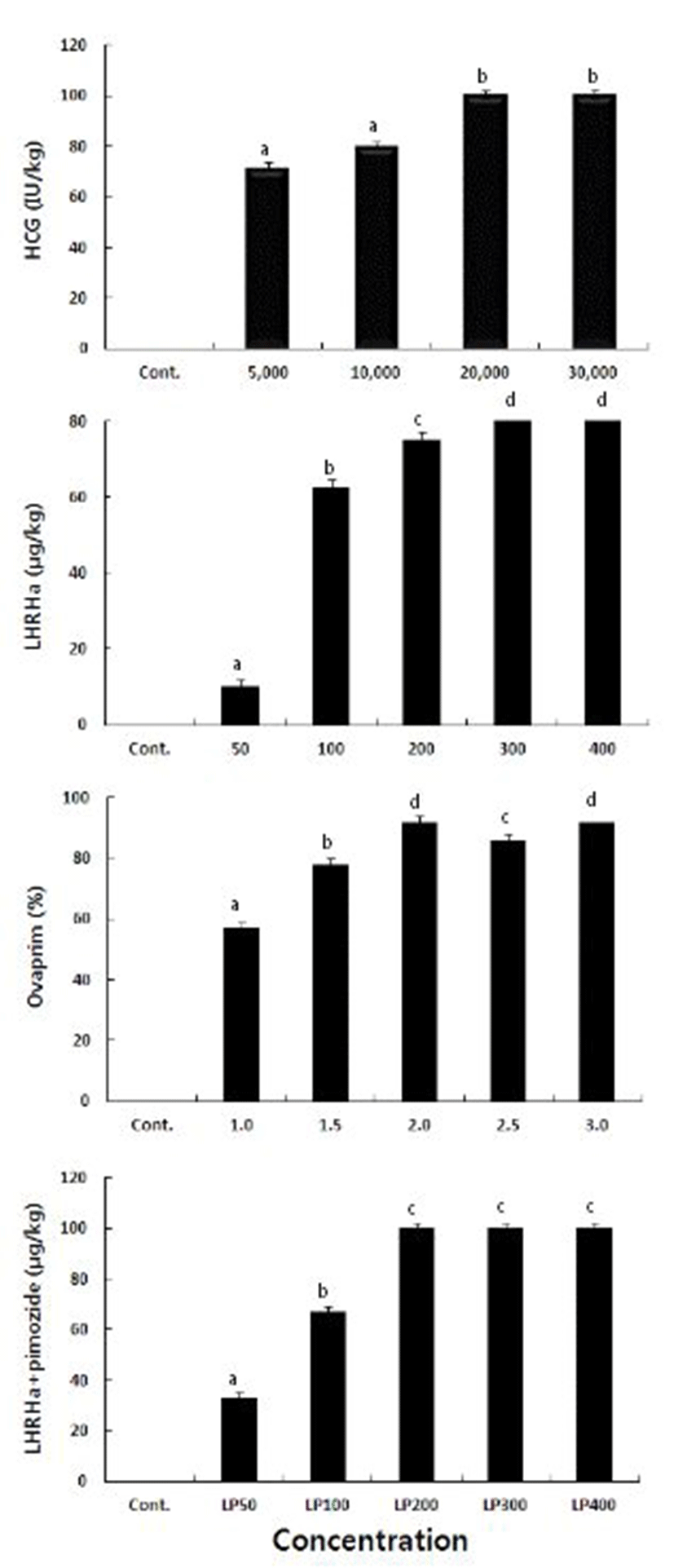
Treatment with hCG of 30,000 IU/kg resulted in the shortest ovulation time of 17 hr. Ovulation time increased as the concentration decreased, as the ovulation times were 19, 18.5, 17.5 at hCG concentrations of 5,000, 10,000, and 20,000 IU/kg, respectively. LHRHa showed a similar pattern as the ovulation times were 34, 28.5, 19.5, and 25 hr at concentrations of 50, 100, 200, and 400 μg/kg. The shortest ovulation time of 16.5 hr was observed at LHRHa concentration of 300 μg/kg. It took, on average, 28.5 hr for the eggs to ovulate at ovaprim of 3.0 ml/kg, and 38, 35, 36, and 32 hr at ovaprim concentrations of 1.0, 1.5, 2.0, and 2.5 ml/kg, respectively, showing the same pattern hCG also showed. When treated with the combinations of LHRHa and pimozide (1,000 μg/kg), at 400 μg/kg the ovulation time was 24.5 hr and was the shortest. At 50, 100, 200, and 300 ml/kg of concentrations, the ovulation times were 33, 28.5, 27, and 25.5, respectively, leading to the common observation of decreasing ovulation time with increasing concentration of any hormone (Table 1).
The ovulation rates at hCG concentrations of 20,000 IU/kg and 30,000 IU/kg are 100%. As it was 71.4% and 80% at 5,000 IU/kg and 10,000 IU/kg, respectively, the ovulation rate tended to be higher at higher concentration. When treated with LHRHa that affects final maturation and ovulation, 100% of ovulation rate was reached at 300 and 400 μg/kg, and at 50, 100, and 200 μg/kg, the ovulation rate was 10, 62.5, and 75%, respectively. Thus it showed the same pattern as hCG. Treatment with Ovaprim resulted in the highest ovulation rate of 92% at both 2.0 and 3.9 ml/kg of concentration and at the concentration of 1.0, 1.5, and 2.5, the ovulation rate was 57, 78, and 86%, respectively. Combinations of LHRHa and pimozide yielded 100% ovulation rate at LHRHa concentration of 200, 300, and 400, and the lowest ovulation rate of 50% at 50 μg/kg (Fig. 1).
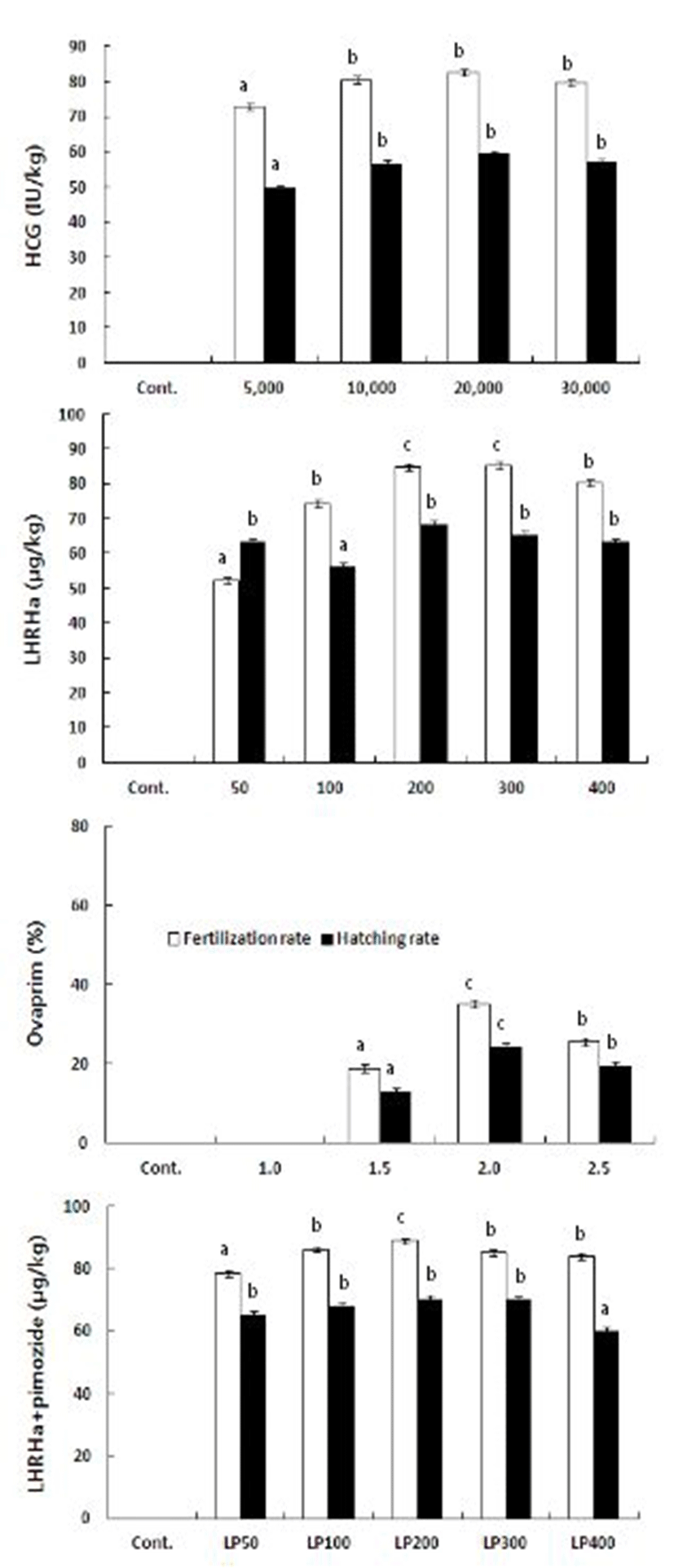
When treated with hCG, the fertilization and hatching rates of 82.7% and 59.4% were the highest at 20,000 IU/kg. The rates were the lowest when 5,000 IU/kg was injected (78.2% and 49.7%) but no significant difference was observed at other experimental groups. When LHRHa was injected, the fertilization rate of 85.3% was the highest at 300 μg/kg and the hatching rate of 68.4% was the highest at 200 μg/kg. The lowest were 52.2% and 56.3% at 50 and 100 μ g/kg, respectively. Treatment with Ovaprim yielded the highest fertilization rate of 88.2% at 2.0 ml/kg and the lowest hatching rate of 58.6% at 1.0ml/kg. In contrast to ovulation rate or ovulation time, the fertilization rate seemed to decrease with increasing concentration. The combinations of LHRHa and pimodize (1,000 μg/kg) resulted in the highest fertilization rate of 88.9% at 200 μg/kg and the lowest hatching rate of 60.2% at 400 μ g/kg, showing the same pattern as ovaprim (Fig. 2).
DISCUSSION
Artificial ovulation and spawning can be induced by controlling external environmental factors such as photoperiod and water temperature, or by injecting pituitary hormones such as hCG, LHRH, GnRH, and pimodize. However, it is known to be hard to induce natural spawning in fish belonging to Bagridae (Lim et al., 1997), and thus study on ovulation induction by hormones, rather than by photoperiod and water temperature regulation, is necessary. Unlike LHRHa or GnRH that indirectly works on pituitary, hCG induces ovulation by directly affecting the reproductive organs. By doing so, hCG becomes the cause of low egg quality and, if the ovulation time is skipped, postmature process is accelerated, affecting the fertilization and hatching rates (Kwon et al., 1996). Kwon’s finding was supported by the results of this study, as the ovulation time is shorter when hCG is injected (16–20 hr) than when LHRHa (23–34 hr), ovaprim (27–40 hr), or the combinations of LHRHa and pimozide (22–36 hr) was injected. But in the study of long snout bullhead, ovulation time was 28–48 hr when hCG was injected twice, 28–44 hr when LHRHa was injected twice, 66 –92 hr when hCG was injected once, 66–86 hr when ovaprim was injected, and 62–86 hr when the combinations of LHRHa and pimozide was injected. As the times for ussurian bullhead are shorter than these results, it can be determined that even for same fish that belong to the Bagridae, different hormones and concentrations of hormones are needed to be adjusted. Also, as fertilization and hatching rates of hCG do not differ too much from other hormones’. This result is not consistent with the results from Lim et al., as Lim founded that, for ussurian bullhead, hCG accelerates ovulation by directly affecting the reproductive organs but it also lowers the quality of the eggs by doing so.
LHRHa is known to be 30–50 times more effective than natural LHRH (Park et al., 1998) and is also known to be effective in ovulation inducement in catfish (Kwon et al., 1996) and bullhead Pseudobagrus fulvidraco (Park et al., 1998). However, as it has been reported that final maturation and ovulation did not occur in the spotted halibut, Verasper variegatus (Baek et al., 2000), it seems like different hormones are effective in ovulation inducement in different species. In this study, too, except for the treatment with 50 μg/kg of LHRHa, the ovulation rate was higher than 62%, fertilization rate was higher than 75%, and hatching rate was higher than 56%, while the ovulation rates were 50% at 200 μg/kg, 75% at 300 μg/kg, and 100% at 400 μg/kg of LHRHa. The positive association between concentration and effectiveness of ovulation was observed and no ovulation occurred at concentrations less than 100 μg/kg. These results were consistent with results from this study (Park et al., 1998).
As for Ovaprim, it has recently been used in ovulation induction in Gobiobotia macroephala (Ko et al., 2011), Pseudopungtungia tenuicorpa (Ko et al., 2012), and in Clarias gariepinus (Sharaf, 2012). In Sharaf’s study, ovulation time of 9.9 hr, ovulation rate of 100%, and hatching rate of 84% were observed when ovaprim was injected to African catfish, while in Lim et al., 2012, ovulation time of 66–86 hr, fertilization and hatching rates of 56.8% and 30% were observed at 2.5 ml/kg of ovaprim in long snout bullhead. Since these rates were the highest, it could be concluded that ovaprim is not suitable in ovulation inducement in long snout bullhead. However, in this study, high ovulation rate of over 80% was observed at concentrations higher than 2.0 μg/kg and fertilization and hatching rates were over 72% and 60%, respectively. This shows that it is less effective than in African catfish but more effective than in long snout bullhead. Also, as the concentration increased from 1.0 to 3.0 ml/kg, the ovulation time shortened from 36–40 hr to 27–30 hr, while the fertilization rate increased, further studies on ovulation inducement at higher concentration of ovaprim would be needed.
The affordability and manageability of pimozide allow it to be used to induce ovulation and to be combined with hCG (Park et al., 1998; Song et al., 2008), GnRHa (Jang et al., 1998), and LHRHa (Park et al., 1998) to be used. In Bagridae, LHRHa by itself can be used for ovulation inducement, but when it is used with pimozide, the ovulation rate gets higher and use of LHRHa can be reduced. Generally, good results can be obtained when pimozide of 1,000 μg/kg and 200– 300 μg/kg of LHRHa are combined. But the ovulation rate of 100% and the highest fertilization and hatching rates of 78% and 60% were obtained at LHRHa concentration of 200 μg/kg in this study and this result does not stand out when compared to other experimental groups.
Therefore, all hormones showed similar results. But as hCG is easy to purchase and is affordable, it can be concluded that hCG is the most useful in obtaining stable fertilization eggs in the most effective way.

