INTRODUCTION
Adipose tissue derived from the embryonic mesenchyme contains a stroma like bone marrow. Adipose tissue can be grown by the deposition of lipid in existing mature fat cells and the production and filling of new fat cells (Wang et al., 1988). Adipocyte precursor replication is accelerated by several pituitary peptides and by some function associated with massive obesity (Roncari et al., 1981).
The regional differences in capacity in adipocyte formation have been evaluated. For example, lipectomy and/or high fat feeding results in greater increases in fat cell number in some depots than in others (Faust et al., 1978). Interregional differences in fat accumulation has been tried to explain through the composition of adipocyte precursor pool, because the pool of adipocyte precursor is an important determinant of the size of adipose tissue. It is suggested that inherent differences in adipose derived mesenchymal cells (adipose derived stem cell; ADSCs) dynamics contribute to the distinct responses of different fat depots to overfeeding (Djina et al., 1983; Tchoukalova et al., 2010). From the various studies, it is revealed that ADSCs express the mesenchymal stem cell markers but not hematopoietic lineage marker and endothelial cell markers (Zuk et al., 2002; Romanov et al., 2005). ADSCs exhibit stable growth and proliferation kinetics and have multilineage potency to the other cell types including osteogenetic, chondrogenetic, myogenic, or adipogenetic lineages in vitro (Izadpanah et al., 2006; Rodriguez et al., 2005; Romanov et al., 2005). Morphologically, ADSCs are fibroblastlike in vitro (Zuk et al., 2002; Zannettino et al., 2008).
Although overfeeding is a caused of increase of the size of adipose tissue, and its related hormonal secretion is the reason of the increase the proliferation of ADSCs, it is not clear how keep the number of cell of each type. Cell-cell contact is known to be a critical regulating signal of cellular proliferation, differentiation and motility. Inhibition of proliferation by density-dependent, cell-cell contact, is generally referred to as contact dependent inhibition of growth or contact inhibition (Eagle et al., 1967). In vitro, non-transformed cells are arrested in G0/G1-phase at a critical cell density forming a confluent monolayer. In adult tissues, contact inhibition is thought to be continuously active, playing a critical role in the repression of somatic cell proliferation and probably organ size control (Zeng et al., 2008). Cell-cell contact inhibition has also meaning of the gradual development of plasma membrane polarity. In line, transformed cells are characterized by a loss of contact inhibition which is manifested by a higher saturation density and the emergence of multi-layered foci in vitro. Despite its importance for cell cycle control, knowledge about the signaling cascade mediating contact inhibition is still scarce (Küppers et al., 2010). Gene expression analysis in mouse fibroblasts revealed that 110 transcripts are differentially expressed in confluent versus proliferating cultures representing 107 genes and 3 cDNA sequences involved e.g. in proliferation, signal transduction, transcriptional regulation, cell adhesion and communication (Küppers et al., 2010).
In vitro, contact-inhibition becomes apparent by the fact that adherent, non-transformed cells stop proliferating at a critical cell density forming a confluent monolayer. In contrast, transformed cells are characterized by loss of contact-inhibition manifested by a higher saturation density and the emergence of multilayered. In an attempt to understand the regulation mechanisms of adipocyte formation, we have studied the cell-cell contact inhibition of subcutaneous adipose derive stem cells. In this study we observed cell morphology, doubling time, and differentiation potency. Gene expression studies comparing 3T3-L1 cell versus msADSCs of various passages were revealed differential expression of genes which are involved e.g. in proliferation, signal transduction, transcriptional regulation, cell adhesion and communication.
MATERIALS AND METHODS
Ten to twelve week-old female CD-1 mice were used in this study. All experimental animal studies followed to the Guide for the Care and Use of Laboratory. Animals were maintained under standard conditions at Sungshin Women’s University. Animals were fed a standard rodent diet and water ad libitum from weaning at 21 days of age. The condition is maintained by 14/10 hr light and dark cycle.
Mouse subcutaneous adipose tissue was obtained from the CD-1 female mice. In briefly, approximately 1.8 g of mouse subcutanous a dipose tissue was washed several times in Hank’s buffered salt solution (HBSS), consisting of 1% BSA, 200 nM adenosine, 50 mg/ml glucose. The adipose tissue was minced finely using surgical scissors and incubated in digestion buffer at 37°C in digestive buffer with constant agitation for 1 hr. The digestive buffer contained 0.1% type I collagenase (Gibco, Cat #. 17100-017) and 1% albumin. After digestion the mononuclear cells were washed and seeded. Cell suspensions were cultured in Dulbecco’s modified Eagle’s medium low glucose (DMEM-LG) (Gibco, Cat #. 31600-026) supplemented with 10% fetal bovine serum (FBS) (Welgene, Cat #. S001-07), 100 U/ml penicillin, 0.1 mg/ml streptomycin, 3.7 mg/ml sodium bicarbonate at 5% CO2, 37°C. All of the nucleated cells were plated at 25,000 cells/cm2 density in 10ml of medium in a culture dish and incubated at 37°C with 5% CO2. After 24 hr, nonadherent cells were discarded, and adherent cells were thoroughly washed twice with PBS. The mADSC was incubated for 5–7 days, harvested with 0.25% trypsin and 1mM EDTA for 1–3 minutes, and replated at 25,000 cells/cm2 density in a culture dish. Medium was changed every other day. To prevent spontaneous differentiation, cells were maintained at subconfluent levels.
As control, mouse 3T3-L1 preadipocyte cell line was used. 3T3-L1 fibroblast cell line was kindly donated by Prof. Lee (Sungshin Women’s University, Korea). The cells were cultures in Dulbecco’s modified Eagle’s medium high glucose (DMEM-HG) (Gibco, Cat #. 12-800-017) supplemented with 10% fetal calf serum (FCS) (Gibco, Cat #. 16170-078), 100U/ml penicillin, 0.1mg/ml streptomycin, 3.7mg/ml sodium bicarbonate at 5% CO2, 37°C. The cells were incubated for 3–5 days, harvested with 0.25% trypsin and 1mM EDTA for 1–3 minutes, and replated at 5,000 cells/cm2 (Low density) and 40,000 cells/cm2 (High density) in a culture dish, respectively. Medium was changed every day. To prevent spontaneous differentiation, cells were maintained at subconfluent levels.
msADSCs and 3T3-L1 cells were maintained in noninductive control meidum until 90–95% confluent the culture plate. The expression profiles of the genes which are related to cell-cell contact inhibition were analyzed. Total RNA from mADSC and 3T3-L1 cells were isolated using TRIzol Reagent according to the manufacturer’s instructions. The purity of RNA was assessed by determining the ratio of absorbance at 260 nm to that at 280 nm. First strand cDNA was synthesized using First-Strand synthesis system (Stratagene, Cat #. 200420) according to the manufacturer’s instructions. Briefly, the mixtures were incubated at 65°C for minutes and lpace tube at room temperature for 10 minutes for the primers to anneal to the RNA. And incubated at 42°C for 60°C minutes and incubated at 70 for 15 minutes to terminate cDNA synthesis. Quantitative real-time PCR was performef for FoxM1, Zeb1, Necl-5, Dusp9, Aurkb, RB1, Itm2b, Ncam1, Tsc-22, Tle2, Tle6, and Hes1. The expression of GAPDH was used as internal control. The primers (Bioneer, Koear) were depicted in Table 1.
mADSC and 3T3-L1 cells were cultured on sterile glass cover slips for a few days until they reached the desired confluency. Cells were washed with PBS and then fixed 4% paraformaldehyde in PBS. After washing with PBS containing 0.1% BSA, cells were blocked with PBS containing 5% normal rabbit serum, 0.3% Triton X-100 in 0.1% BSA/PBS for 30 minutes at RT. Between each step, cells were washed with 0.1% BSA in PBS. Cells were incubated with anti- Actin polyclonal primary antibody (Goat polyclonal IgG, Santa Cruz Biotechnology) (1:500) overnight at 4°C. After labeling with the primary antibody, cells were then incubated with Cy3 conjugated rabbit anti- goat IgG were used as the secondary antibody (Cy3 conjugated affinipure Rabbit Anti-Goat IgG, Jackson) (1:200) for 1 hour at RT. And then cells were counterstained with Hoechst33258 for 20 minutes at RT. Immunoreactive cells were visualized by fluorescence microscopy.
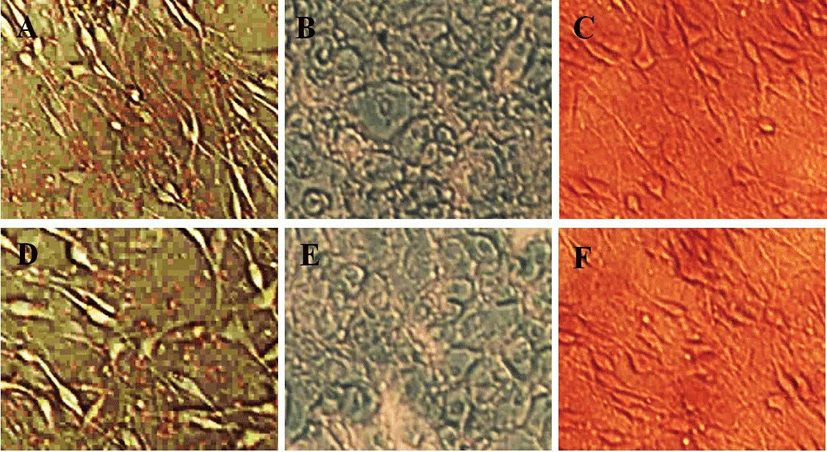
Adipogenic differentiation was induced and assayed according to Zuk protocols. Passage 5, 10 and 15 ms-ADSCs were induced. The acquisition of the adipogenic phenotype was determined by staining the monolayers with 2% Oil Red-O solution. To assess the osteoblastic phenotype, 2% Alizarin red staining was used to assess matrix mineralization after 21 days. For condrogenic differentiaiton, the cells were stained with 1% alcian blue. We chose 10 visual fields randomly, and analyzed the positive products of three populations of each passage.
RESULTS
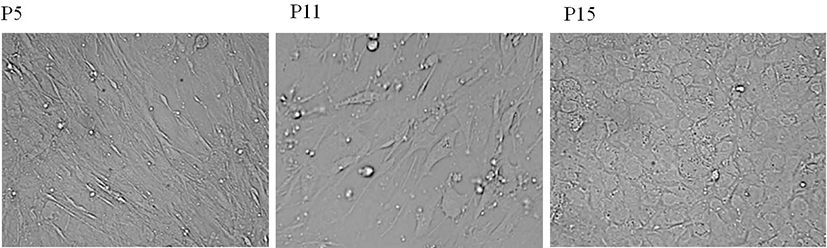
Most case of cell shape change means the functional change. During passage, the morphological changes of msADSC were analyzed. During the passage msADSCs were kept fibroblast-like shape, belly shape (Fig. 1A, B). After passage 14, however, the morphology of cells was changed. msADSCs were angular squamous shape with clear boudary between cells (Fig. 1C). The morphology of msADSCs was not reversible after passage 14.
Because the morphology of msADSCs was changed during maintaining the lineage, we analyzed whether the doubling times changed after passage 14. Under the seeding density in this study, the doubling time was not different between p1, p15, and p25. The mean time of doubling was 52.3±8.378 hr. The change of the morphology was not cause of delay of cell proliferation (Fig. 2).
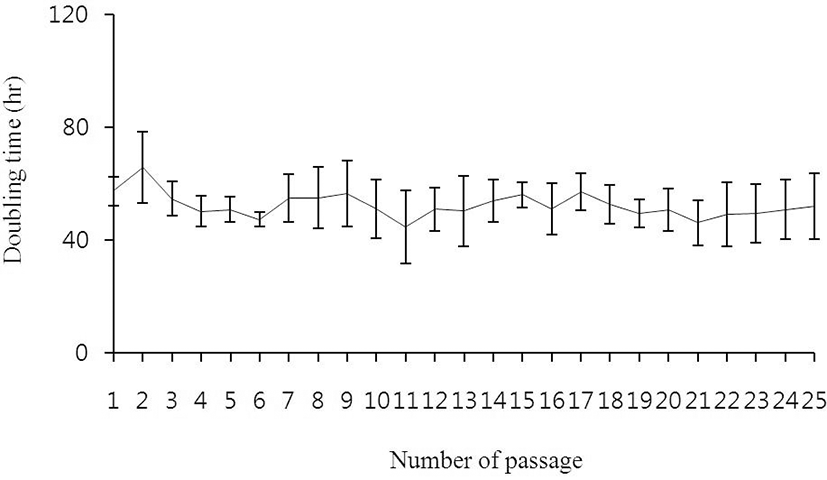
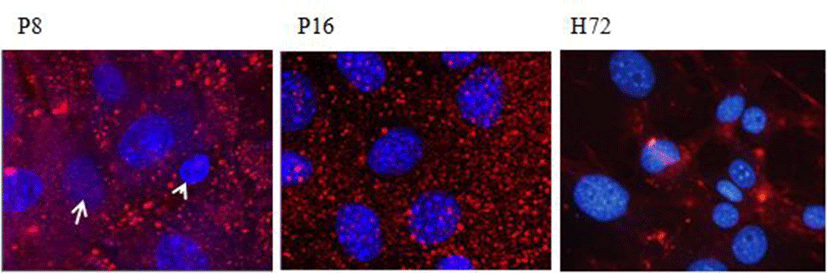
Cell-cell contract proliferation inhibition is evaluated from early 1960s. It is known that such a proliferation inhibition also exist in stem cell. During early passage we observed that some of the cells showed overlapping growth. After culture for 15 days the msADSCs were applied to confocal microscope. The XYZ images were got and detected the localization of nucleus. Interestingly, nucleus formed the stratified layers in the fibroblast-like shaped msADSCs (Fig. 3A). On the other hand, after passage 14 the cell shapes were changed, the msADSCs of passage 15 did not form stratified nuclear layer (Fig. 3B). 3T3-L1 cell was used as control of cell-cell contact inhibition of proliferation and showed same pattern with that of passage 15 (Fig. 3C).
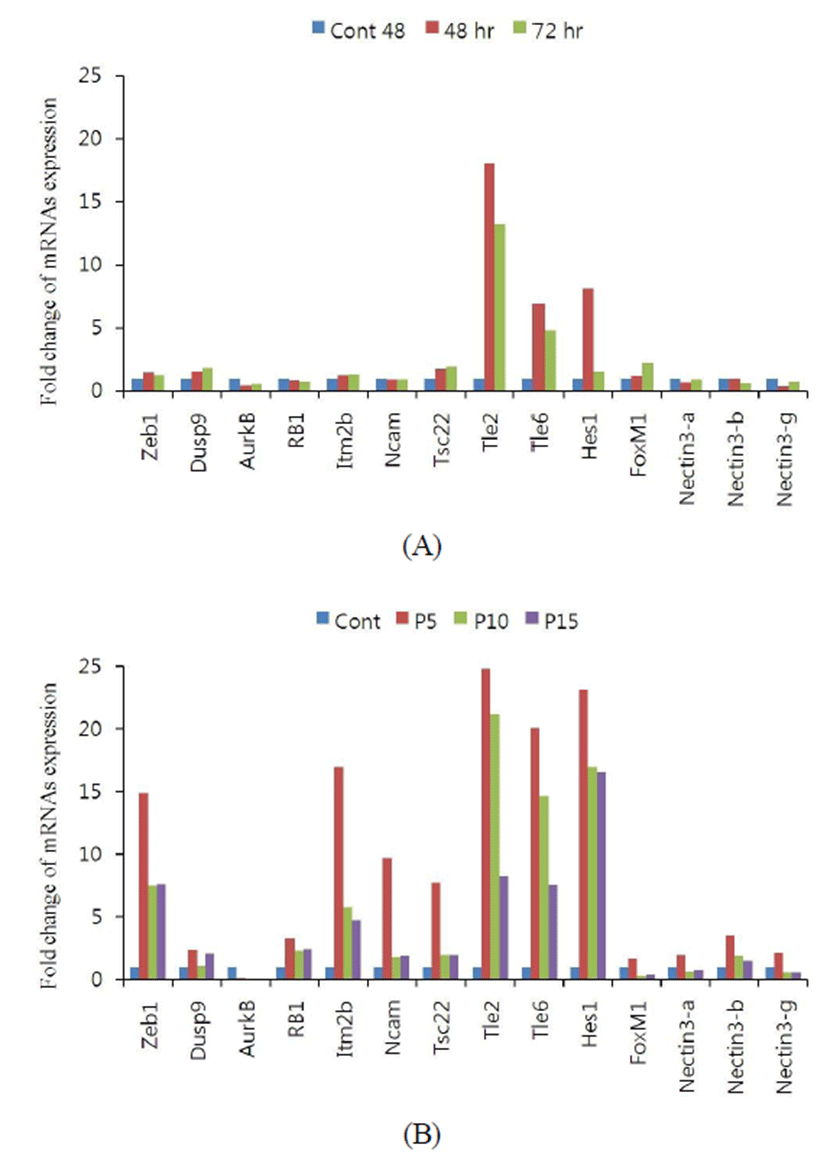
To confirm the change of cell-cell contact inhibition of proliferation, the expression of genes which were known to involve in contact inhibition was analyzed. The kind of genes which are known to involve in cell-cell contact inhibition are various. In 3T3-Li, hairy and enhancer of split-1 (Hes1) gene expression is essential in contact inhibition of cell proliferation by repressing E2F-1 (Noda et al., 2011). At 48 hr of high density seeding, the cells occupied about 90% of the plate and the mRNA expression levels of Hes1 gene was dramatically increased. The mRNA expression levels of transducin-like enhancer protein 2 and 6 (Tle2, Tle6) were also dramatically increased. However, the expression levels of Zeb1, Dusp9, AurkB, RB1, Itm2b, Ncam, Tsc22, Foxm1, nectin3-alpha gene, nectin3-beta gene, nectin3-gamma gene were not changed by the density increase (Fig. 4A). In msADSCs, the expression patterns were different for that of the 3T3-L1. The mRNA expression levels of Zeb1, Rb1, Itm2b, Ncam, Tsc22, Tle2, Tle6, Hes1, and nectin3-beta genes were high more than 4 fold in msADSC on passage 5. On passage 10 msADSCs, the mRNA expression levels of Zeb1, Itm2b, Ncam, Tsc22 and nectin genes were decreased but the levels were not same with those of passage 15. On passage 15 msADSCs, the mRNA expression levels of Zeb1, Itm2b, and Hes1 were similar with those of pass 10 but other genes expression levels were decreased dramatically (Fig. 4B).
These results mean that the cell-cell contact inhibition was increased after morphological change. To evaluate whether the morphological changes could decrease the potency for differentiation, the msADSCs of passage 5, 10, and 15 were induced to adipocyte, condrocyte, and osteocyte. As seen in Fig. 5, the adipogenic, chondrogenic, and osteogenic differentiation potency were not different between passage 10 and passage 15.
DISCUSSION
The polyclonal nature of adipocyte precursor population has been observed with respect to replication (Wang et al., 1988). After then ADSCs is a new source of mesenchymal stem cells and a good materials in various field of medicine. The culture methods to get sufficient stem cells from in vitro culture have been developed, because it is needed the huge number of cell for tissue repair. For example about 4.0 million human mesenchymal stem cells (hMSC) are need for a single injection aimed at infarcted heart repair (Seger & Lee, 2008). It is known that there is contact inhibition in MSCs and passage after reaching 70–90% confluence to prove cell proliferation. Therefore, to get huge number of MSCs, we needed passage. Lineage maintaining of MSCs are usually get through passage and it cause of the lose pluripotency and proliferation capacity (Bruder et al., 1997; Javazon et al., 2004; Vacanti et al., 2005). This is one of the obstacles in apply to the industry and medicine.
From this study it was revealed that the morphology of msADSCs change after passage 14. Fibroblast-like shape become squamous and did not reversible. Such morphological changes were also reported in human ADSC during lineage maintaining. The morphology of human ADSC altered markedly at passage 15 and passage 20, demonstrating the appearance of flattens features compared at early passages (Safwani et al., 2012). Similar features have been reported in mesenchymal cells (Izadpanah et al., 2006). The potency of human ADSCs for adipose tissue genesis and osteogenesis also decrease after passage 15 and passage 20 (Wan Safwani et al., 2011). So it is suggested that human ADSCs beyond passage 10 may not be suitable to used clinically as their safety and efficiency may be compromised (Safwani et al., 2012). The different between msADSC and human ADSC of Safwani et al (2012) is that the morphological changes of human ADSC happed at passage 15 and passage 20 but only in passage 15 in msADSC. Interestingly the differentiation potency of msADSC was not decreased by long-term culture (passage 15). In addition, the doubling time also was not different between passage 5, passage 10, and passage 15, and until passage 25. Although further studies are needed, the reasons of difference may be come from the regional difference between msADSC and huamn ADSCs of Safwani et al. (2012).
Preadipocyte such as 3T2-L1 is a well know cell line which has cell-cell contact inhibition. Also proliferation of mesenchymal stem cells becomes contact inhibited (Krinner et al., 2010). It is known that the proliferation of human ADSC is also blocked by cellcell contact inhibition in vitro. On the other hand, it has been suggested that the properties of accumulation of adipocyte are regional specific characters. Population derived from anatomically separate regions differs with respect to the frequency of clones of varying replicative capacities (Wang et al., 1988). Lower body fat-cell progenitors can develop rapidly into mature adipocytes in adult humans in response to overfeeding and that this response depends partially on sex and baseline adipocyte size (Tchoukalova et al., 2010). The number of leg fat cells is greater in overweight than in normoweight persons (Tchoukalova et al., 2008). Besides, by the amount of nutrient and the physiological condition, the growth of fat tissue is observed. Those mean that the adipose stem cells can proliferate without less limitation responding to the external condition. In addition, interestingly, in vitro culture of mouse subcutaneous adipose derived stem cells showed the growth patterns of stratification. These results evaluate the possibility that the cell-cell contact inhibition is weak in msADSC compared with the other cells like 3T3-L1.
The expression profiles of some genes which have function in cell-cell contact inhibition in msADSCs were different from 3T3-L1 cells. The expression levels of Zeb1 were dramatically high compared with 3T3-L1 in passage 5 msADSCs. And its high expression levels were detected on passage 10 and passage 15 msADSCs. It is known that Zeb1 express in the condition of loss of cell-cell contact (Liu et al., 2009). On the other hand, a few of genes which are known that highly expressed in the cell proliferation suppressed cells were detected in msADSCs of passage 5, 10, and 15 like in confluent 3T3-L1 cell. These are including Hes1 (Noda et al., 2011), Tle2, Tle6 (Evans et al., 2008), Tsc22 (Küppers et al., 2010). Cell-cell contact inhibition results in the inhibition of proliferation and locomotion (Ben-Ze’ev et al., 1980; Nakamura et al., 1983). Interestingly, the expression profiling data showed that the genes known in loss or gain cell-cell contact inhibition were expressed in msADSCs but not in 3T3-L1. The expression profiles of the genes of passage 20 msADSCs were similar with those of passage 15 msADSCs. Although further studies are needed, it is suggested that the difference of the expression profiles of these genes are the cause of the character of msADSCs.
In conclusion, msADSC was characterized by the getting strict cell-cell contact inhibition and morphological changes after passage 14 but the proliferation was not blocked by the change of that in vitro. Besides the msADSC of passage 15 keep the differentiation potency like msADSCs of passage 5 or 10. It is needed further studied to evaluate the physiological meaning of the occurring cell-cell contact inhibition during in vitro culture of mADSC. The meaning of expression of the genes involved in cell-cell contact inhibition also needed to evaluation. It is suggested that the understanding of cell-cell contact proliferation inhibition in ADSCs may be a first step to understanding the developmental role in adipose tissue generation.







