INTRODUCTION
Notch signaling is used in a variety of cellular contexts during animal development to specify distinct cell fate among individual cells. In vertebrate embryos, Notch signaling is important for the formation of central nervous system (CNS), proliferation of the undifferentiated neural progenitor cells, and generating a variety of neural cell types (Latimer & Appel, 2006; Artavanis-Tsakonas & Muskavitch, 2010). In Drosophila, the sensory organ precursor cells develop from a group of equipotent neurogenic epithelial cells, which use Notch signaling to select neural progenitor cells (Campos-Ortega, 1995; Lai, 2004). The neural progenitor cells again use Notch signaling to specify different cell fates following asymmetric cell division. The asymmetric cell division gives rise to a pIIa cell in which the Notch signaling becomes activated (non-neural fate) and a pIIb cell in which this signaling remains inactive (neural fate). The difference in Notch signaling activity is established by Numb (Uemura et al., 1989; Guo et al., 1996). In this process, Numb protein is asymmetrically segregated and localized to the pIIb daughter cell, in which suppresses Notch signaling activity. Numb encodes an adaptor protein that is able to bind the Notch receptor and interacts with endocytic proteins such as alpha-adaptin and EPS15, and then functions to promote the pIIb cell fate (Santolini et al., 2000; Berdnik et al., 2002). Recently, it was reported that Numb inactivates Notch by promoting its endocytosis during asymmetric cell division in Drosophila embryos (Couturier et al., 2012). Interaction of Numb with the Notch signaling pathway appears to be conserved in animal development, particularly in specification of the neural precursor cells (Kandachar & Roegiers 2012).
Ascidians belong to the Urochordata. They are one member of the phylum Chordata with vertebrates and cephalochordates. The ascidian tadpole larvae possess a typical chordate body plan with a CNS composed of only ~100 neurons (Meinertzhagen et al., 2004). The simplicity of ascidian CNS makes it an ideal model for understanding the development and function of chordate-specific neuronal networks. Notch signaling is also involved in specification of the neural progenitor cells and neural tube patterning in ascidian embryos. Overexpression of Notch led to defects in neural tube closure and in the formation of brain vesicle, palps, and peripheral neurons (Akanuma et al., 2002). It was also reported that a Delta2/Notch-mediated relay from the posterior motor ganglion specifies the fate of anterior motor ganglion (Stolfi et al., 2011). During ascidian embryogenesis, however, function of Numb in Notch signaling remains unknown.
In this study, we isolated and characterized the ascidian Numb gene. The ascidian Numb transcript was found to be expressed both maternally and zygotically. Its zygotic expression is observed in dorsal neural precursor cells from the early neural stage embryo. Larvae injected with Numb morpholino oligonucleotide showed abnormalities in brain and palps formation, but not pigment cells.
MATERIALS AND METHODS
Adults of the ascidian Halocynthia roretzi were collected or purchased from fishermen in the vicinity of the Marine Biology Center for Research and Education at Gangneung-Wonju National University, Gangneung, Korea. Naturally spawned eggs were fertilized with a suspension of sperm from another individual, and then raised in filtered seawater containing 50 μg/mL streptomycin sulfate and 50 μg/mL kanamycin sulfate at 10-13°C. Embryos were collected at appropriate stages and fixed for whole-mount in situ hybridization.
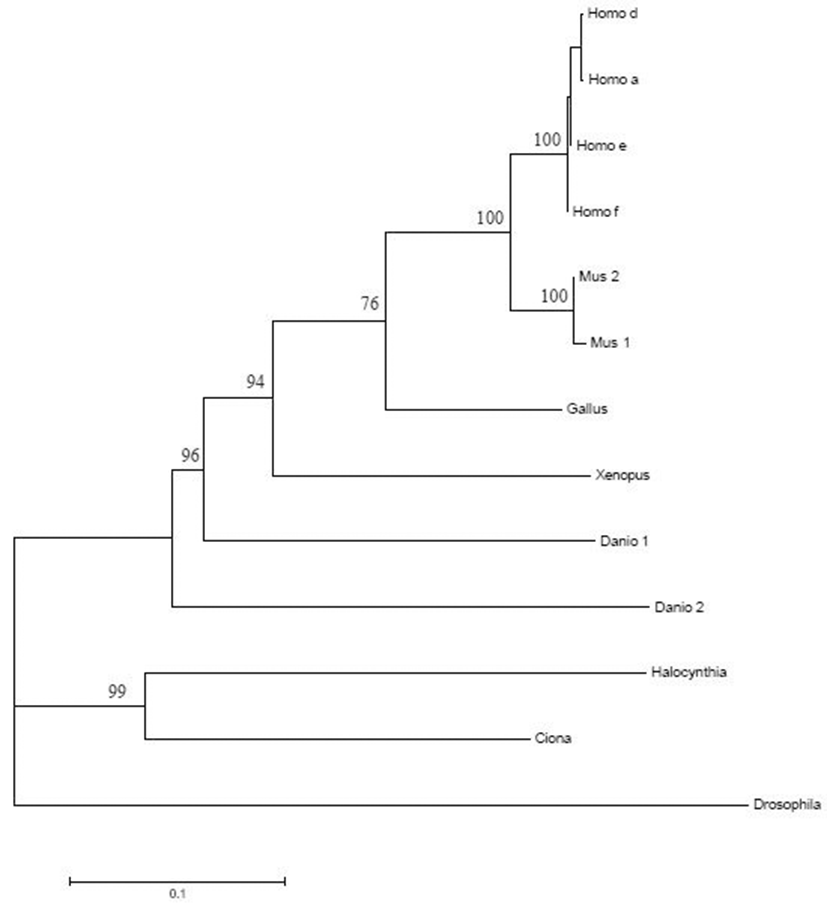
The cDNA fragments encoding a part of N-terminus region of Halocynthia Numb were isolated by RT-PCR with degenerate oligonucleotides, 5’-CA(A/G)TGGCA(A/G) (A/C/G)(C/A)IGA(C/T)GA(A/G)G-3’ for the upstream, 5’-(G/A)AAIGC(G/A)CAICCIACIGC(G/A)T-3’ for the downstream, and 5’-(A/G)GTI(A/T)(G/C)ITT(C/T)TG(C/T) GCICCIGA-3’ for the nested downstream primers from gastrula stage poly(A) RNA. Larger fragments of Hr-Numb covering the complete ORF were obtained by 5’ and 3’ rapid amplification of cDNA ends (RACE) using a SMART RACE cDNA amplification kit (Clontech). The primers for RACE were the following: 5’-GTCGTGATGG AACTACGAGACGCTGGATATG-3’ for the upstream and 5’-GGCTTAAACGTTCACCCGAATCCTTAATGGC-3’ for the downstream. Molecular phylogenetic relationships among the Numb products were estimated with MEGA 5.05 program using the neighbor-joining method (Saitou & Nei, 1987). Sequence data used in this study were taken from GenBank databases, with following accession numbers: Homo a, Homo sapiens Numb isoform CRA-a (EAW81100.1); Homo d, Homo sapiens Numb isoform CRA-d (EAW81110.1); Homo e, Homo sapiens Numb isoform CRA-e (EAW81104.1); Homo f, Homo sapiens Numb isoform CRA-f (EAW81114.1); Mus 1, Mus musculus Numb1 (AAD47835.1); Mus 2, Mus musculus Numb2 (AAD47836.1); Gallus, Gallus gallus Numb (AAD49434.1); Xenopus, Xenopus tropicalis Numb (CAL49325.1); Danio 1, Danio rerio Numb (AAT85677.1); Danio 2, Danio rerio Numb-like (AAI07954.1); Ciona, Ciona intestinalis Numb (BAE06599.1); Drosophila, Drosophila melanogaster Numb isoform A (AAF52776.1).
Whole-mount in situ hybridization was performed using a digoxigenin-labeled Hr-Numb antisense probe, as described previously (Miya et al., 1997; Lee et al., 2011). Specimens were hybridized with the probe at 50°C.
To suppress functions of Hr-Numb, we injected the morpholino antisense oligonucleotide (MO, Gene Tools) into eggs as described by Kim et al. (2007). The nucleotide sequence of Hr-Numb MO was 5’-GCTTTGTCTTAT TGTCCTTATCATG-3’. The standard control MO provided by Gene Tools was used as a control experiment. MOs were dissolved in sterile distilled water with Fast Green and injected into fertilized eggs. The final concentration of each MO to be injected was approximately 1 mg/mL. The injected eggs were allowed to develop up to hatching stage.
RESULTS AND DISCUSSION
To clarify the mechanism by which Notch signaling specify neural cells during ascidian embryogenesis, we isolated Numb homologue (Hr-Numb) in the ascidian Halocynthia roretzi by the classical RT-PCR and 5’ and 3’ RACE. In various Numb proteins, the N-terminal region, which contains the phosphotyrosine-binding (PTB) domain interacting with a wide variety of different proteins, is highly conserved among vertebrates and invertebrates (Berdnik et al., 2002; Reugels et al., 2006). Using degenerate oligonucleotide primers (see Materials and Methods), we amplified target fragments from the Halocynthia gastrula stage poly(A) RNA by RT-PCR. Then, a fragment of Halocynthia Numb covering the complete ORF was obtained by RACE. A cDNA clone for Hr-Numb was 1,624 base pairs in full length, and the predicted protein has 456 amino acids (Fig. 1). The predicted amino acid sequence contained a well-conserved N-teminus sequence between various Numb homologues. Although the over degree of amino acid identity was not high (about ~ 30%), the amino acid sequence of Hr-Numb PTB domain was highly conserved when compared with the PTB domains of other Numb proteins (data not shown).
In order to understand the relationships among the Numb genes from various animal groups, we assembled a molecular phylogenetic tree (Fig. 2). The phylogenetic tree clearly shows that Hr-Numb is an Halocynthia orthologue of vertebrate Numb. Numb-like gene (e.g. Danio 2 in Fig. 2) is a related member of the Numb family of endocytosis promoting genes (Niikura et al., 2006). Numb and Numblike share high sequence similarities at amino acid level. However, Numb-like has two specific motifs that were not found in Numb, in the N-terminal and middle regions (Zhong et al., 1997; Niikura et al., 2006). Hr-Numb did not contain these motifs, suggesting to be an orthologue of Numb rather than that of Numb-like. There is no report that Ciona intestinalis, another ascidian species, has a Numb-like gene.
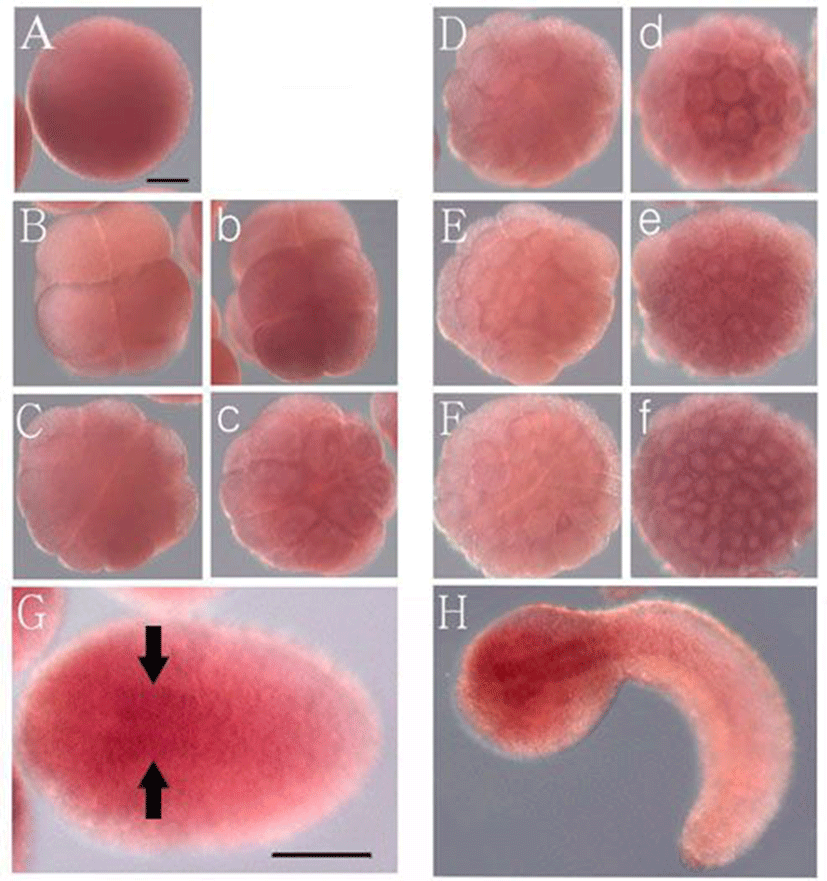
Whole-mount in situ hybridization demonstrated that the maternal transcripts of Hr-Numb are distributed evenly in the egg cytoplasm (Fig. 3A). Cleavage of Halocynthia eggs is bilaterally symmetrical. At the 8-cell stage, the transcripts were distributed in the cytoplasm of the animal pole-side blastomeres, although the signal was weak in the vegetal pole-side blastomeres (Fig. 3B, b). This expression pattern was inherited by the early gastrula stage. The maternal transcripts became concentrated near the cytoplasm of the animal blastomeres (Fig. 3c-f). Zygotic expression of Hr-Numb was firstly observed in the dorsal neural precursor cells at the early neurula stage (Fig. 3G, arrows). At the tailbud stage, the expression became strongly in the A- and a-lineage neural precursor cells of the dorsomedial head region (Fig. 3H). These cells give rise to brain, peripheral neurons, and palps. The expression pattern of Hr-Numb is similar to that of Hr-Notch reported by Hori et al. (1997). Hr-Notch was maternally expressed and its zygotic expression was visible in the dorsal part of the A-lineage nerve cord precursor, and in the a-line brain and palps precursor cells at the neurula stage. Thus, it is suggested that Hr-Numb is involved in Notch signaling in which specify the anterior region of neural precursor cells during ascidian embryogenesis. The expression pattern of Hr-Numb is also similar to that of zebrafish Numb. In zebrafish, Numb transcripts were detectable in all regions of the embryos at the mid-blastula transition stage (Reugels et al., 2006). Expression of zebrafish Numb was found in the midline from the head to the tail regions at the neurula stage, and then the signal was restricted in the brain and eye progenitor cells at the later stage. Therefore, it is likely that roles of Numb protein are conserved in Notch signaling, which specify neural precursor cells in chordate development.
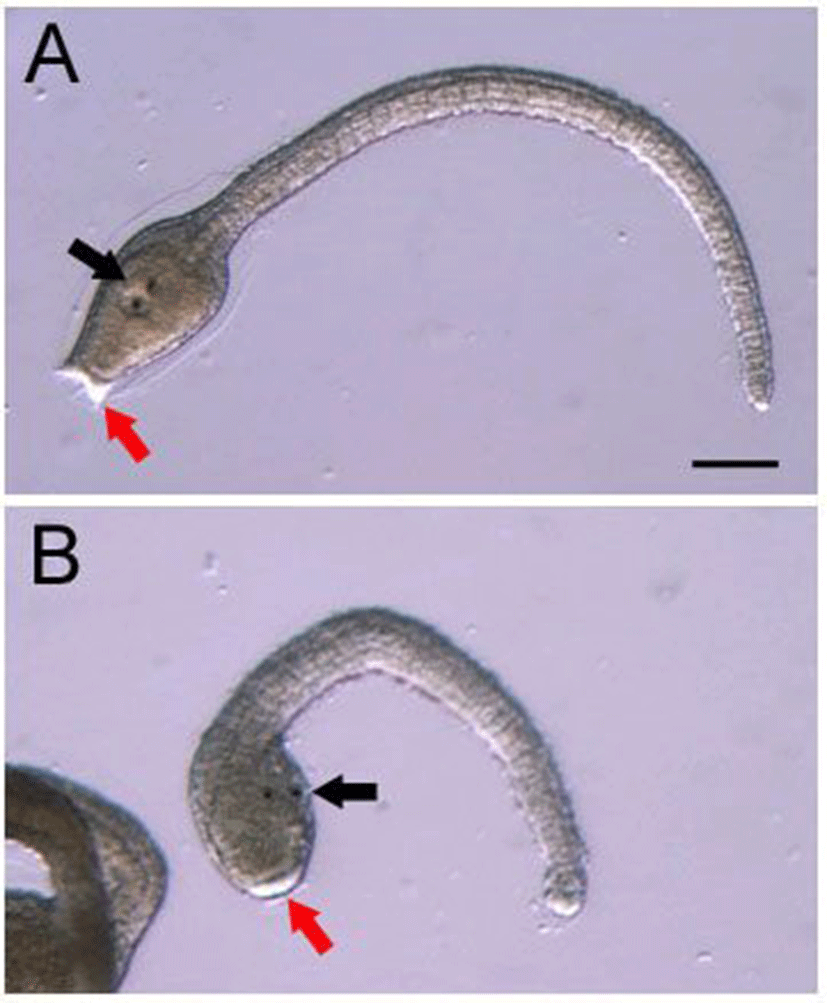
Next, we attempted to inhibit functions of Hr-Numb by injecting fertilized eggs with antisense morpholino oligonucleotide (MO). Antisense MO prevents translation of the coding region of the targeted mRNA. The timing of initiation of gastrulation was delayed about one hour in the Hr-Numb MO injected embryos (85%, n=47) compared with normal embryos. Injection of Hr-Numb MO resulted in the loss of brain vesicle (Fig. 4B, black arrow) and palps (Fig. 4B, red arrow) in 89% (n=47) of cases. However, the Hr-Numb MO-injected larvae showed formation of pigment cells, although the cells located outside of head region (Fig. 4B, black arrow). This might be caused by incomplete neural tube closure. No specific abnormality was observed in the larvae injected with control MO in 91% of cases (n=32) (Fig. 4A). These results suggest that Hr-Numb is essential for brain vesicle and palps formation, but not for pigment cells formation. Similar results have been reported by Akanuma et al. (2002). Overexpression of the constitutively activated Hr-Notch forms resulted in larvae with defects in neural tube closure and brain vesicle formation. The activated Hr-Notch also suppressed formation of palps, but not pigment cells. Therefore, it is plausible that Hr-Numb is involved in control of Notch signaling during ascidian embryogenesis.
In zebrafish, Numb and Numb-like are involved in differentiation of primitive erythrocytes (Bresciani et al., 2010). The zebrafish Numb and Numb-like knockdown experiments showed severe reduction or absence of embryonic erythrocytes. There is a report that Notch signaling controls the Numb protein level. In the developing chick CNS, a reciprocal negative regulation presents between Notch and Numb proteins, namely, high levels of Notch activation cause a reduction in the Numb and Numb-like protein levels (Chapman et al., 2006). Inhibition of Notch signaling by Numb is essential for various cell fate decisions. Future studies using Hr-Numb may provide new insights for Notch signaling during ascidian embryogenesis.

