INTRODUCTION
The trophoblast constitutes an undifferentiated single layer of cytotrophoblast forming the outer layer of a blastocyst and the multinucleated double later of syncytiotrophoblast that is fused from cytotrophoblast at the period of embryo implantation (Huppertz, 2008). Multinucleated syncytiotrophoblasts come into contact with villous basement membrane and differentiate into villous syncytiotrophoblasts that proliferate with a fibroblast growth factor-4. Those differentiated cells form syncytial knots, induce cell death, and are released through placental blood vessels (Scifres & Nelson, 2009). Also, cytotrophoblast can differentiate into another type of trophoblasts called extravillous trophoblasts. These are formed by an invasion of trophoblastic cell columns into the uterine stoma. Extravillous trophoblast stem cells in the cell columns penetrate into the myometrim and can differentiate into the endovascular extra villous trophoblast and interstitial extra villous trophoblast forming a normal placenta. This process provides an adequate blood supply to the placenta with spiral arteries and facilitates the exchange of nutrients, wastes, and gases between the maternal and fetal systems. This proliferation, differentiation, and adequate cell death play a significant role to maintain placental development. Inadequate trophoblast cell deaths can induce trophoblast dysfunction by lowering trophoblast numbers resulting in abnormal placentation and several gynecological diseases (Huppertz et al., 2006; Na et al., 2010).
Immortalization-upregulated proteins (IMUPs) are novel genes identified in SV40-immortalized human fibroblast cell lines, encoding two novel nuclear proteins, IMUP-1 (321 bp, 10.9 kDa) and IMUP-2 (258 bp, 8.5 kDa). IMUP-1 and IMUP-2 arise during transcriptional processing through an RNA alternative splicing procedure (Kim et al., 2000; Ozer, 2000; Kim et al., 2008). In addition, IMUPs influence cellular distortion, proliferation, cell death, and tumorigenesis including lungs, colon, and ovarian cancer (Kim et al., 1998; Kim et al., 2003). Although IMUPs are known for their cellular proliferation and cell death, however, trophoblastic effectiveness is not well known. A recent study showed placentas with an obstetrical disease, such as pre-eclampsia, have an excess of IMUPs compared with normal placentas and the excess IMUPs can cause cell death (Jeon et al., 2010).
Programmed cell death is classified as necrosis, apoptosis (Type I programmed cell-death), and autophagy (Type II programmed cell-death) (Bildirici et al., 2012). Apoptosis is the process of programmed cell death that may occur in a homeostatic context of the cell organisms or during growing morphology. The genetically engaged survival process plays an important role in physiological development, homeostasis, and immunity facilitating appropriate growth and differentiation for the normal placental development (Lockshin & Zakeri, 2004). Conversely, autophagy is the catabolic mechanism that maintains the turnover rate of organelles. It involves the cell degradation of unnecessary cellular components through lysosomal machinery or ubiquitin-like conjugation reactions, which fuses the isolated cells with lysosomes. Recent studies show that the autophagosome formation occur through a nucleation complex of Class III PI3K and Beclin from Bcl2 as well as from the reaction of LC3-II and Atg5-12 complexes that are independently generated protein conjugation cascades (Singh, 2011). Another study showed that expression of LC3 II was in fact more elevated in cesarean section placentas than in vaginal deliveries. Also, the expression of LC3 II was increased in pre-eclamptic placentas than in normal placentas (Oh et al., 2008). However, how IMUP-2 expression changes affect cell death and functions are not clear. Therefore, the objective of study is to analyze the effect of IMUP-2 on apoptosis and autophagy in trophoblast cell.
MATERIALS AND METHODS
HTR-8/SVneo trophoblastic cells at the early stage of pregnancy were provided by Dr. Charles H. Graham (Queen’s University, Canada). They were exposed to an atmosphere consisting of 5% CO2 at 37°C maintained in RPMI-1640 medium (Gibco BRL) supplemented with 100 U/ml penicillin and streptomycin (Gibco BRL, NY, USA), 5% fetal bovine serum (FBS, Gibco BRL), and 100 U/ml penicillin/streptomycin (Gibco).
To extract plasmid DNA containing IMUP-2 genes, E. coli containing IUMP-2 plasmid was incubated for 16 to 18 hours using rotator shaking and a HiYield Plasmid mini kit (RBC Bioscience Corp, Taipei, Taiwan) was used to extract pcDNA3/IMUp-2 from E. coli. HTR-8/SVneo trophoblast cells (1×106) were seeded onto 100 mm culture dishes (Thermo Scientific, MA, USA). When the cells reached 70% confluence, they were mixed with 500 ng pcDNA3/IMUP-2, 1 nM siRNA/IMUP-2, and 2 μl Lipofectamin 2000 (Invitrogen, CA, USA), exposed at room temperature for 20 minutes. They were switched to Opti-MEM (Gibco BRL), a reduced antibiotics and serum media. Then they were transfected with a 500 ng of pcDNA3/IMUP-2 and 1 nM siRNA/IMUP-2 to be cultured for 24 hours.
We conducted a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) analysis to analyze cell death, depending on the expression of IMUP-2, with pcDNA3/IMUP-2 and siRNA/IMUP-2 transfected cells. 1×104 HTR-8/SVneo trophoblast cells were seeded onto 96-well plates (BD Biosciences, California, USA), transfected with 500 ng of pcDNA3/IMUP-2 and 1 nM of siRNA/IMUP-2 using 2 μl of Lipofectamin mix, then cultured for 24 hours to perform the MTT analysis. 20 μl of MTT liquid with 5 mg/ml density was added and maintained at 37°C for 2 hours and 30 minutes after MTT liquid was lysed. Then, 200 μl of dimethyl sulfoxide (DMSO) was added. To test reaction, they were placed on a gyratory shaker for 5 minutes. Next, optical densities were measured with a wavelength of 562 nm.
To detect the change of IMUP-2 expression in trophoblast cells with pcDNA3/IMUP-2 and siRNA/IMUP-2 transfection, a RT-PCR analysis was performed. The total amount of RNA was extracted using RNeasy RNA isolation kit (QIAGEN, California, USA) and Trizol reagent (Invitrogen). The extracted RNA densities were quantified by a Nano Drop spectrophotomer (Thermo Scientific). 1 μg of the extracted total RNA, 0.5 μg of oligo dT, 10mM dNTP mix (Invitrogen), and DEPC-D.W (Invitrogen) were blended, and the transcription reaction was performed at 65°C for 5 minutes. Afterwards, the transcription reaction was performed at 50°C for an hour and at 72°C for 15 minutes using a 5× first-strand buffer, 0.1 m DTT, RNase-out, and 200 U/μl of superscript III RT (Invitrogen) to synthesize cDNA. The synthesized cDNA was amplified by PCR, using 20 pmol/μl of IMUP-2 primers and a H-Taq polymerase kit (Solgent Co., Ltd, Daejon, South Korea). PCR products were visualized by electrophoresis on a 1.2% (w/v) agarose gel, and the amplified DNA was indicated by a quantity video image analyzer (Bio-Rad, CA, USA). Then the housekeeping genes of the 28s rRNA bands were quantified using the Quantity One program to conduct a comparative quantitative analysis on the degree of expression of each DNA group with the control group of IMUP-2. The sequences for IMUP-2 primer used in the study are 5'- ATG GAG TTC GAC CTG GG-3' (Forward), 5'-ACT TCA CAT CCG TG TCCG-3' (Reverse), and the sequences for 28s rRNA primer are 5'-TTG AAA ATC CGG GGG AGA G-3' (Forward), 5'-ACA TTG TTC CAA CAT GCC AG-3 ' (Reverse).
To analyze how IMUP-2 expression affects the cellular proliferation, and cell death, we performed a western blot analysis on the expression of the correlated factors of cell cycle, apoptosis, and autophagy. 24 hours after transfecting pcDNA3/IMUP-2 and siRNA/IMUP-2 into the trophoblast cells, protein was lysed in 200 μl RIPA buffer (50 mmol/l Tris-HCl, ph 7.6, 150 mmol/l NaCl, 1% NP40, 0.25% deoxycholic acid, 0.1% SDS, 1 mmol/l EGTA, 1 mmol/l EDTA, 10 mmol/l NaF, 1 mmol/l NA3VO4) containing a protease inhibitor cocktail (Roche, Basel, Switzerland). Protein extracts were quantified by BCA assay kit (Pierce, Texas, USA). The total of 40 μg protein extracts were heated at 95°C for 5 minutes, loaded onto 8 to 15% sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) for 2 hours, and electrotransferred onto PVDF membranes (Bio-Rad) at 70V. The membranes were blocked by TBS-T, made from TBS (Abel Bio, Seoul, South Korea) containing 5% albumin serum bovine (BSA; Amresco, Ohio, USA) and 0.1% Tween 20 (Sigma-Aldrich, Missouri, USA), for 80 minutes and then reacted with various antibodies overnight at 4°C (Table 1). The next day, after washing them with TBS-T, we tested their reaction at room temperature for an hour using horseradish peroxide (HRP)-conjugated rabbit (1:25,000) (Bio-Rad) or anti-mouse lgG (1:25,000) (Bio-Rad) secondary antibodies. The protein was detected by visualizing peroxide activity using an ECL advance western blotting detection kit (Amersham Bioscience, Buckinghamshire, UK).
To explore the effect of IMUP-2 expression on trophoblastic apoptosis, caspase activities in pcDNA3/ IMU-2 and siRNA-IMUP-2 transfected trophoblastic cells were analyzed using a Capsase- Glo 3/7 assay kit (Promega Corp, Wisconsin, USA). The cell lysates were quantified using a protease inhibitor-free lysis buffer (Culture Lysis Reagent; Promega), 50 μg of protein, and reacted solution, placed in wells, were exposed to the ordinary temperature for 2 hours and 40 minutes. Fluorescence of the reacted samples was measured with a wavelength of 470 nm using a SpectraMax L microplate reader (Molecular Devices, California, USA). Also, to indicate the fragmented DNA from apoptosis, after washing incubated trophoblastic cells on glass cover slip (Nunc, FL, USA) with PBS, the slides were fixed in methanol (Merck, New York, USA) at room temperature for 10 minutes. They were stained with 1 μg/ml PI (Sigma) at room temperature for 5 minutes to detect nuclear segmentation, washed, and mounted in glycerol-based mounting solution (DAKO, Glostrup, Denmark). Then, they were observed with an EVOS fl microscope (AMG, Washington, USA).
The pcDNA3/IMUP-2 and siRNA-IMUP-2 transfected trophoblastic cells were treated with 0.05% of trypsin and TrypLE Express solution (Gibco BRL) containing 1mM of EDTA. The extracted cells were immersed in 100% ethanol (Merck) at room temperature for 10 minutes and in 0.5 μg/μl RNase A (Sigma) solution for 15 minutes. Then they were stained with Propidium Iodide (PI) solution (1:200, Sigma). The cycle of stained cells was examined using a FACScan flow cytometer (BD Biosciences).
The pcDNA3/IMUP-2 and siRNA-IMUP-2 transfected trophoblastic cells were incubated on 24-well plates with glass cover slips (Nunc) for 24 hours, washed with PBS (Sigma), and then mounted in 100% methanol (Merck) for 15 minutes. The cells were blocked by protein block serum-free buffer (Dako) at room temperature for 30 minutes. Rabbit anti-IMUP2 (provided by Jinkyoung Kim, CHA University, Korea, 1: 100×) for IMUP-2 expression analysis and rabbit anti-human LC3 (Norvus Biologicals, CO, USA, 1:200x) for the detection of autophagosome formation were separately incubated overnight at 4°C. The next day, after washing them with PBS, they were subsequently incubated in a humidified chamber with a secondary antibody conjugated to Alexa Fluor 488 donkey anti-rabbit IgG (H+L) (Invitrogen, 1:400) at the ordinary temperature for 30 minutes. Then, they were counterstained in DARI-contained mounting solution (Dako) and analyzed using an EVOS fl microscope (AMG). To detect autophagosome using MDC staining, the 24 hours incubated trophoblastic cells were fixed in 4% paraformaldehyde solution (Usb Corporation, Ohio, USA) after being reacted with 0.05 mM MDC (Sigma) at 37°C for 30 minutes. They were mounted in fluorescence mounting medium (Dako) and were observed with an EVOS fl microscope (AMG).
RESULTS
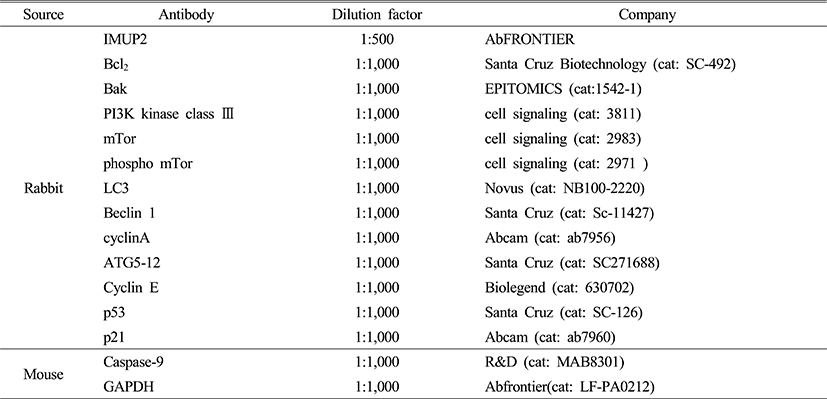
To detect the change of IMUP-2 expression with pcDNA3/IMUP-2 and siRNA/IMUP-2 transfection in HTR-8SV/neo trophoblasts, reverse transcription polymerase chain reaction (RT-PCR) analysis was performed. We observed that quantitative mRNA expression of IMUP-2 significantly increased in the pcDNA3/IMUP-2 transfected cells but decreased in siRNA/IMUP-2 transfected trophoblastic cells (p<0.05, Fig. 1A, B). Similarly with the RT-PCR result, the protein expression of IMUP-2 increased in the pcDNA3/IMUP-2 transfected trophoblastic cells when compared with mock but decreased in siRNA/IMUP-2 transfected trophoblastic cells (Fig. 1C). Also, when observed with immunofluorescence for IMUP-2 expression in HTR-8/SVneo trophoblastic cells, the cellular localization of IMUP-2 was translocalized from cytoplasm into nuclear in pcDNA3/IMUP-2 transfected trophoblasts, comparing to the Mock group, but no nuclear translocalization was observed in siRNA/IMUP-2 transfected trophoblasts (Fig. 1D).
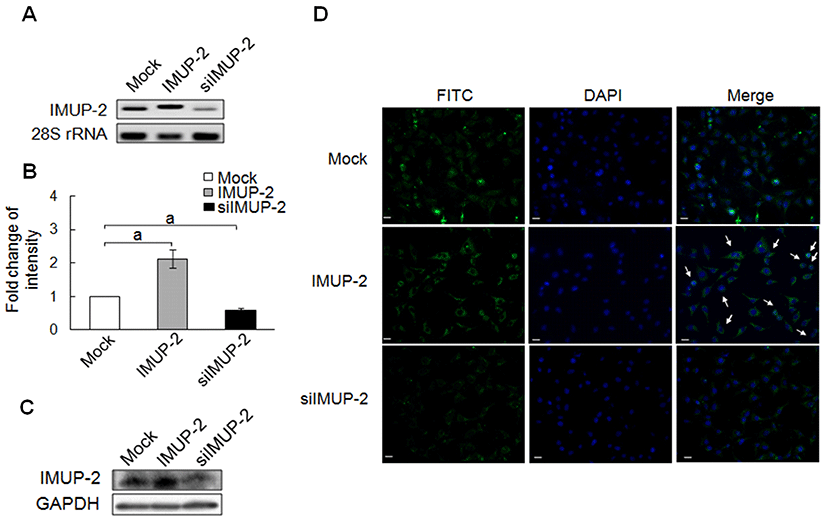
In cell cycle analysis using FACS analysis, generally, the sub G1 phase of trophoblasts were increased by IMUP-2 expression through up and down--regulation of IMUP-2 (p<0.05, Fig. 2A). However, the sub G1 phase of pcDNA3/IMUP-2 transfected trophoblasts shows a tendency to increase more than siRNA/IMUP-2 transfected trophoblasts (p<0.05). In case of the S phase, there are no statistical significances in pcDNA3/IMUP-2 expression, although they increased from 13.44% to 16.54% compared to the mock control. However, we observed statistical significance in the S phase of siRNA/IMUP-2 expression showing the increase from 13.44% to 19.37% (p<0.05, Fig. 2A). Also, for the G2/M phase, there is no difference although pcDNA3/IMUP-2 expression decreased from 27.65% to 25.85% comparing to the mock control. However, siRNA/IMUP-2 expression, compared to the mock control, showed significant decrease from 27.65% to 21.00% in G2/M phase than pcDNA3/IMUP-2 (p<0.05, Fig. 2A).
Next, we analyzed the expression of cell cycle-related genes to determine the IMUP-2 effect. The expressions of cell cycle-related gene such as Cyclin A and Cyclin E in siRNA/IMUP-2 treated tropholbasts were increased comparing to the mock group. However, the expression of p53, which is a checkpoint of the cell cycle, did not show significant difference even though it slightly increased in both cells of pcDNA3/IMUP-2 and siRNA/IMUP-2 when compared to the mock. Also, the expression of p21 generally decreased in both pcDNA3/IMUP-2 and siRNA/IMUP-2 comparing to the mock, but siRNA/IMUP-2 transfected cells decreased more than the pcDNA3/IMUP-2 transfected cells (Fig. 3C). The results suggest that the increased expression of IMUP-2 can induce apoptosis. But, the decreased expression of IMUP-2 is associated with cellular proliferation although the expression of IMUP-2 seems to be involved in cell death.
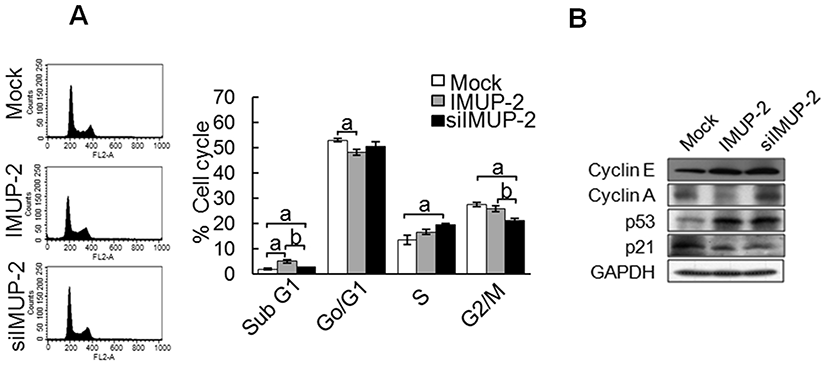
From the MTT analysis on cellular proliferation depending on the IMUP-2 expression in trophoblasts, we observed that the cell numbers of pcDNA3/IMUP-2 transfection were more significantly decreased than those of siRNA/IMUP-2 (Fig. 3A, Fig. 3B, p<0.05). Also, we analyzed other apoptosis-related factors to explain the reason for cell number decrease. The representative proapoptotic markers such as Bak and caspase-9 were increased in pcDNA3/IMUP-2 transfected trophoblasts, but the expression of Bcl2 as an anti-apoptosis marker did not show a significant difference. However, the expressions of Bak, caspase 9 and Bcl2 were decreased in siRNA/IMUP-2 transfected trophoblasts (Fig. 3C). Caspase 3/7 activities showed the highest activities in the pcDNA3/IMUP-2 transfected trophoblasts (p<0.05), but the least activities in the siRNA/IMUP-2 transfected trophoblasts (Fig. 3D). Additionally, to analyze the morphological characteristics on trophoblastic apoptosis, prophodium iodide (PI) staining was used. The results show that excessive expression of IMUP-2 induces nuclear condensation, abnormal phenotypes of the nucleus, and a number of nuclear fragmentations, but relatively decreased expression of IMUP-2 reduces morphological changes in the nucleus (Fig. 3E). These results mean that excessive expression of IMUP-2 induces apoptosis of trophoblast through increased caspase 3/7 activities and abnormal morphological changes comparing to those of down-regualted IMUP-2.
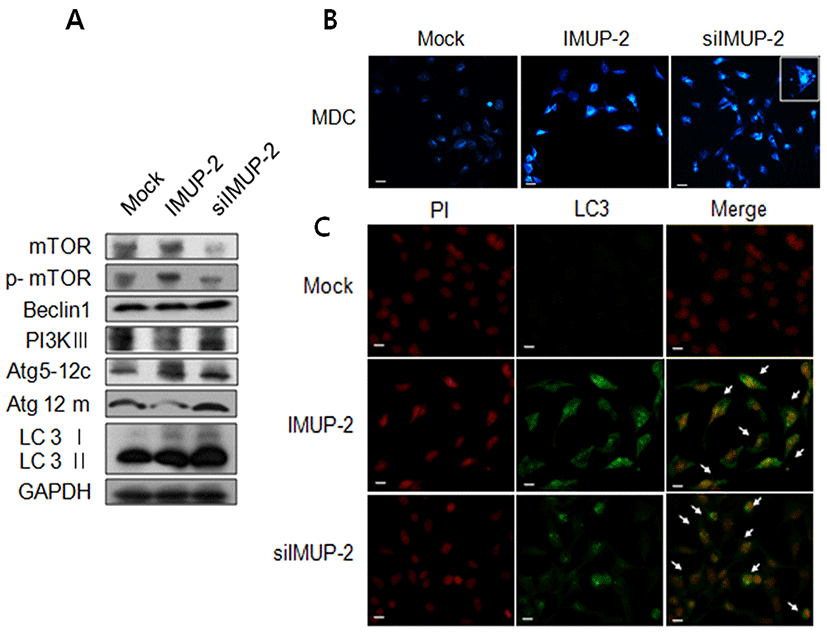
Autophagy, another different form of programmed cell death, was analyzed to determine its fuction on the trophoblasts depending on the IMUP-2 expression. The expression of mTOR phosphorylated protein as an anti-autophagy marker was increased in pcDNA/IMUP-2 transfected trophoblasts comparing to the mock, otherwise, they was significantly decreased in siRNA/IMUP-2 transfected trophoblasts. Furthermore, we observed that the expressions of PI3KIII, ATG 5-12c, ATG 5-12m, LC3 I, and LC3 II were increased in siRNA/IMUP-2 transfected trophoblasts (Fig. 4A). Therefore, to investigate whether autophagy is activated by alternative expression of IMUP_2 in trophoblast, we performed an immunofluorescence assay evaluating LC3, which is an early marker for autophagosomes. Additionally, we employed MDC, which is a fluorescent compound that becomes incorporated into the autophagosomal vacuoles. As shown in Fig. 4B, immunofluorescence also revealed the presence of many cup-shaped and ring-like structures in both types, which represent autophagosomes. We confirmed that the formation of autophagosomes with the MDC incorporation assay in siRNA/IMUP-2 transfected trophoblasts more increased than pcDNA/IMUP-2 transfected trophoblasts (Fig. 4B). Also, the expression of LC3 II was more increased in siRNA/IMUP-2 transfected trophoblasts than cDNA/IMUP-2 transfected trophoblasts.
DISCUSSION
The placental development at the early stage of pregnancy is carried out through balance between the cellular proliferation, differentiation, and adequate cell death of trophoblast (Smith et al., 1997b). During hypoxia condition (~20 mmHg) at the time, trophoblast differentiates into cell layers resulting induce cellular proliferation and oxygen supply as well as apoptosis of the trophoblast (Ray et al., 2010). During placental development, many factors induce cell death were found (Ray et al., 2009). A recent study showed that anti-apoptotic factors in Bcl2 groups slow cell cycle, but pro-apoptotic factors in Bcl2 groups facilitate the cell cycle (Ray et al., 2010). Especially, Bak was reported to involve the cellular proliferation by controlling the cell cycle, but it turned out to be more related to cell death than to the cell cycle (De Falco et al., 2001). The single layer of mononuclear villous cytotrophoblast fuses between cells to differentiated into syncytiotrophoblast, which does not maintain cell proliferation (Hughes et al., 2004). Therefore, excessive apoptosis in syncytiotrophoblast causes negative effects such as decrease in adequate number of trophoblasts during pregnancy, degradation in functioning leading to damage on fetal growth with placental dysfunction and cause gynecological diseases such as preeclampsia or intra-uterine growth restriction (IUGR) (Smith et al., 1997a; Tomas et al., 2011). Therefore, a delicate balance between proliferation and apoptosis of trophoblasts plays a significant role in maintaining a healthy placenta and fetus.
The expression of IMUP-2 correlates with cellular proliferation and oncogenesis in various cancer cell lines. Its overexpression in NIH/3T3 cells acts as an important factor to activate the cellular proliferation, increasing levels of S and G2/M phase cells and inducing p53 pathwayrelated mutations, which involves DNA formation, cell cycle control, and MAK kinase cascade (Ryoo et al., 2006). However, increased IMUP-2 by hypoxia induced apoptosis in trophoblasts of placenta. In previous reports, we demonstrated that IMUP-2 mRNA was expressed in syncytiotrophoblasts, syncytial knots, and decidual cells of the placental villi, however, IMUP-2 mRNA expression was strong in syncytiotrophoblasts and syncytial knots within pre-eclamptic placentas, which induced apoptosis of the trophoblast (Jeon et al., 2010). Syncytial fusion by caspase 8, 9, and 10 activities in cytotrophoblasts and the formation of syncytial knots in syncytiontrophoblasts has been reported to induce apoptosis of trophoblasts (Huppertz et al., 1998; Huppertz et al., 1999). So, we analyzed cellular proliferation in trophoblasts and programmed cell death including apoptosis and autophagy to detect the fluctuation in IMUP-2 expression under the normal oxygen tension because there has been a lot of controversy on the roles of IMUP-2 in the balance between proliferation and cell death.
From the FACS analysis, we confirmed that the G2/M phase was decreased in both pcDNA3/IMUP-2 transfected trophoblastic cells and siRNA/IMUP-2 treated cells (p<0.05), but, the S phase was only increased in siRNA/IMUP-2 treated cells (p<0.05). In addition, cyclin E of cell cycle forms a complex with cdk2, involved with the process from G1 phase to S phase. This complex is inhibited by p21CIP, p27KIP, p57KIP2 in CIP/KIP group among CDK regulators. Also, p53-independent p21 function was reported to be involved in cellular proliferation and normal development in organelles (Tamura et al., 2007). In this study, the similar results were observed showing the increased expression in p53 and p21 in pcDNA3/IMUP-2 cells and the increase of p53 and decrease of p21 can facilitate the cellular proliferation in siRNA/IMUP-2 cells.
Excessive expression of IMUP-2 in trophoblasts increased the activities of caspase 3, 7, and 9, which indicates apoptosis in trophoblasts occurs through two pathways of initiator stages and execution stages. Anti-apoptotic Bcl-2 protein in a placenta at the early stage of pregnancy appears in cytotrophoblasts inhibits autophagy, thereby functioning as a cell proliferators (LeBrun et al., 1993). Also, our data showed that cell death occurred in both pcDNA3/IMUP-2 cells and siRNA/IMUP-2 cells, but the difference lies in that apoptosis by the activities of caspase 3, 7, and 9 were proactive with increased IMUP-2, but on the contrary, decreased IMUP-2 degraded the activities of Bak and Bcl2 meaning cell death by the activities of caspase 3, 7 and 9 can be controllable.
Autophagy is a mechanism that involves cell growth, cellular proliferation, and tumorigenesis through phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) /mTOR pathway (Nicholson & Anderson, 2002). Mammalian target of rapamycin (mTOR), in particular, inhibits autophagosome formation by initiating kinase activity and dysfunctioning sub-cellular targets working essentially in the early stage of trophoblasts (Roos et al., 2009). In this study, it was predicted that excessive expression of IMUP-2 would induce mTOR increase and Class III PI3K decrease leading to inhibit autophagosome formation, but MDC expression and LC3 also turned out to induce autophagosome formation. However, decreased IMUP-2 expression decreases mTOR significantly and increases the expressions of Beclin, Class III PI3K, LC3 II, and Atg5‐12, and facilitates autophagosome formation in MDC and LC3 II staining analysis.
In conclusion, the alteration of IMUP-2 in trophoblasts can control the balance between apoptosis and autophagy of trophoblast by acting as a apoptosis factor when the increased IMUP-2 expression in trophoblasts and also as a autophagy factor to facilitate cellular proliferation when decreased IMUP-2 expression reducing p21 but activating cycline A and E. Therefore, these results will help further studies on functional involvement of IMUP-2 in placental development and gynecological diseases.