INTRODUCTION
The class Amphibia contains vertebrate animals that typically live on land, originally evolved from a form between a fish and a reptile which spread throughout the world (Green & Sessions, 1991; Maxson, 1992; Zug, 1993). Taxonomically, it is classified into about 4,500 different species under 4 orders: Gymnophiona, Meantes, Caudata and Anura (Goin et al., 1978; Duellman & Trueb, 1986; Maxson, 1992; Duellman, 1993; Zug, 1993).
Spermatogenesis refers to a periodic process involving a series of somatic cell and metotic divisions, along with complicated cell maturation and differentiation for the production of haploid cells from polyploid cells (Franca et al., 1999; Weinbauer & Wessels, 1999; Lee & Mōri, 2004). Therefore, such cycles include a series of complete, highly structured and successful cellular associations in a parti-cular given area of the seminiferous tubule, for which the productive system is comprises of multiple stages (Leblond & Clermont, 1952; Clermont, 1972; van Haaster & de Rooij, 1993; Franca et al., 1999; Mizukami et al., 2001; Paula et al., 1999).
Spermatogenesis in amphibians is controlled by external factors, including endogenous rhythm, photoperiod, habitat, etc. (Paniagua et al., 1990), which can vary according to the weather (Cobellis et al., 2002; Chieffi & Minucci, 2004). Studies on external factors such as temperature and photoperiod are on-going, with examination of amphibian reproduction in an environment customized to each species around the summer season (Rastogi et al., 1976; Pierantoni et al., 1984; Paniagua. 1990; Pudney. 1995).
Amphibians form sperm especially at the beginning of the breading season (Chieffi et al., 2000). Breeding takes place from early spring to late summer. The favored con-ditions for embryonic development (Ko et al., 1998) and the optimal external factors, such as temperature, photo-period, water level, etc., vary according to species; therefore, each species has a different set of favorable conditions for breeding (Denver, 1998).
After emerging from hibernation, the testicular cells in amphibians are controlled by hormone or cellular signal delivery systems, stimulated by change of the external environment (Lecouteux et al., 1985; Varriale et al., 1986; Bremner et al., 1994). Around breeding season, the pri-mordial germ cell, type A spermatogonia, initiates reproduction (Raucci & Di Fiore, 2007) and then completes sperm formation prior to hibernation (Ko et al., 1998). Therefore, the formation of amphibians sperm is highly dependent on the climate conditions of the habitat and species. Ultimately, observation of the structural changes in the testis via cellular analysis is required, particularly focusing on the division of reproductive cells upon testicular division (Kera & Iwasawa, 1981; Yoneyama & Iwasawa, 1985).
In amphibian, the testis is divided into the seminiferous lobule and interlobular tissue. In the seminiferous lobule, germ cell cysts are formed by the gathering of germ cells in the same division stage, wherein the production and division of germ cells takes place. Each germ cell cyst is composed of germ cells at a specific division stage (Pudney, 1995). The mature sperm in the cyst is then discharged to the lumen through spermiation (Grier, 1992). The classification of phase of seminiferous epithelium in the sperm formation process becomes a useful tool to study the various factors affecting the regeneration of the spermato-gonium, as well as for analysis of the structural and cellular development, and the division of reproductive cells (Clemont, 1972). In addition, the generation and type of sperm formation, along with the formation of repro-ductive cells occurs in various stages depending on the species, which provides taxonomically useful information (Franca et al., 1999). Stage division of the seminiferous epithelium has been performed on numerous mammals so far, whereas the studies on anurans were performed on Rana esculenta (Rastogi et al., 1976), Rana catesbeiana (Yoneyama & Iwasawa, 1985; Go & Lee, 2001), Bufo japonicus formosus (Moriguchi & Iwasawa, 1987) and R. perezi (Delgado et al., 1989). Therefore, this study was performed to investigate the division stage of reproductive cell through the seminiferous epithelium cycle in Bombina orientalis and further investigate its differentiation from other anural amphibian type.
MATERIALS AND METHODS
Five male Bombina orientalis collected from April to May 2012 near Hwawangsan, located in Changnyeong, Gyeongnam, Korea, were used in this study. The subjects were transferred to the laboratory immediately after collection, and then anesthetized with ethyl ether for extraction of the testis. The extracted testis was deposited in a small closed container containing an aqueous solution of 3%-Glutaraldehyde (4°C, pH 7.4, Millong's buffer) at 4°C for about 2 hours. The testicular tissue was then cut to the thickness of 1~1.5 mm3 and then pre-fixed in an aqueous solution of 3%-Glutaraldehyde (4°C, pH 7.4, Millong's buffer) again. The pre-fixed tissues were washed in a buffer solution (4°C, pH 7.4, Millong's buffer) 3 times. After washing, post-fixation was carried out with 1.33%-OsO4 (pH 7.4, Millong's buffer) for 2 hours, after which they were washed with the same buffer solution as prior to the dehydration at increasing ethanol concentrations (from 70 to 100%). The dehydrated tissues were embedded in Epon 812 mixed solution. An ultramicrotome (MT-6000, Sorvall, Dupont) was used on the embedded tissues to obtain serial sections of 400 nm, which were then dyed with 0.5%-toluidine blue. Each serial stage of the seminiferous epithelium was observed under an optical microscope.
RESULTS
The division stage of the seminiferous epithelium in Bombina orientalis were based upon the cellular morpho-logical characteristics, with division into a total of 10 stages. Cellular division was observed in the following order: from primary spermatogonia to secondary spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids and sperm. Germ cell cysts at the same stage were attached to the seminiferous epithelium, and the sperms which fell out from the germ cell cyst were discharged into the inner seminiferous tubule (Table 1, Figs 1-10).
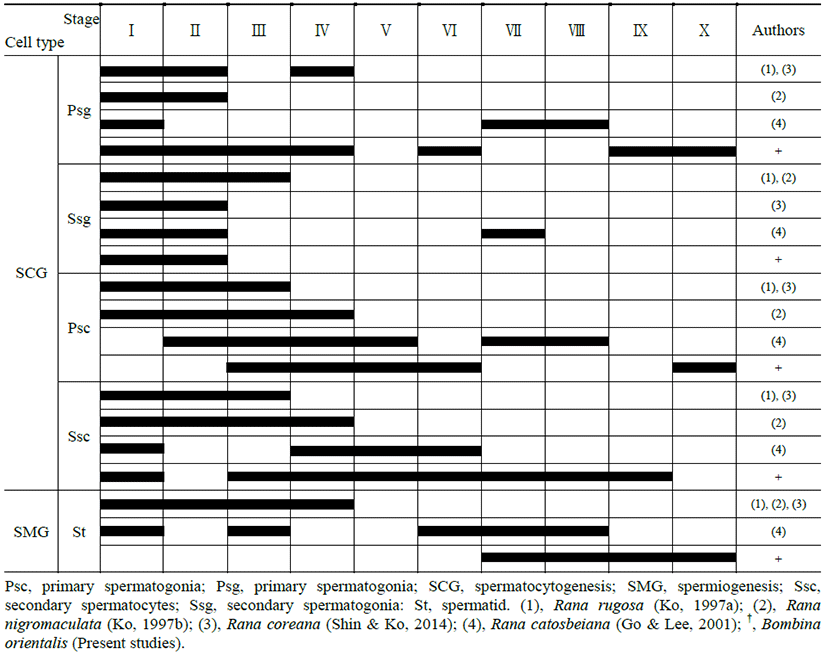
|
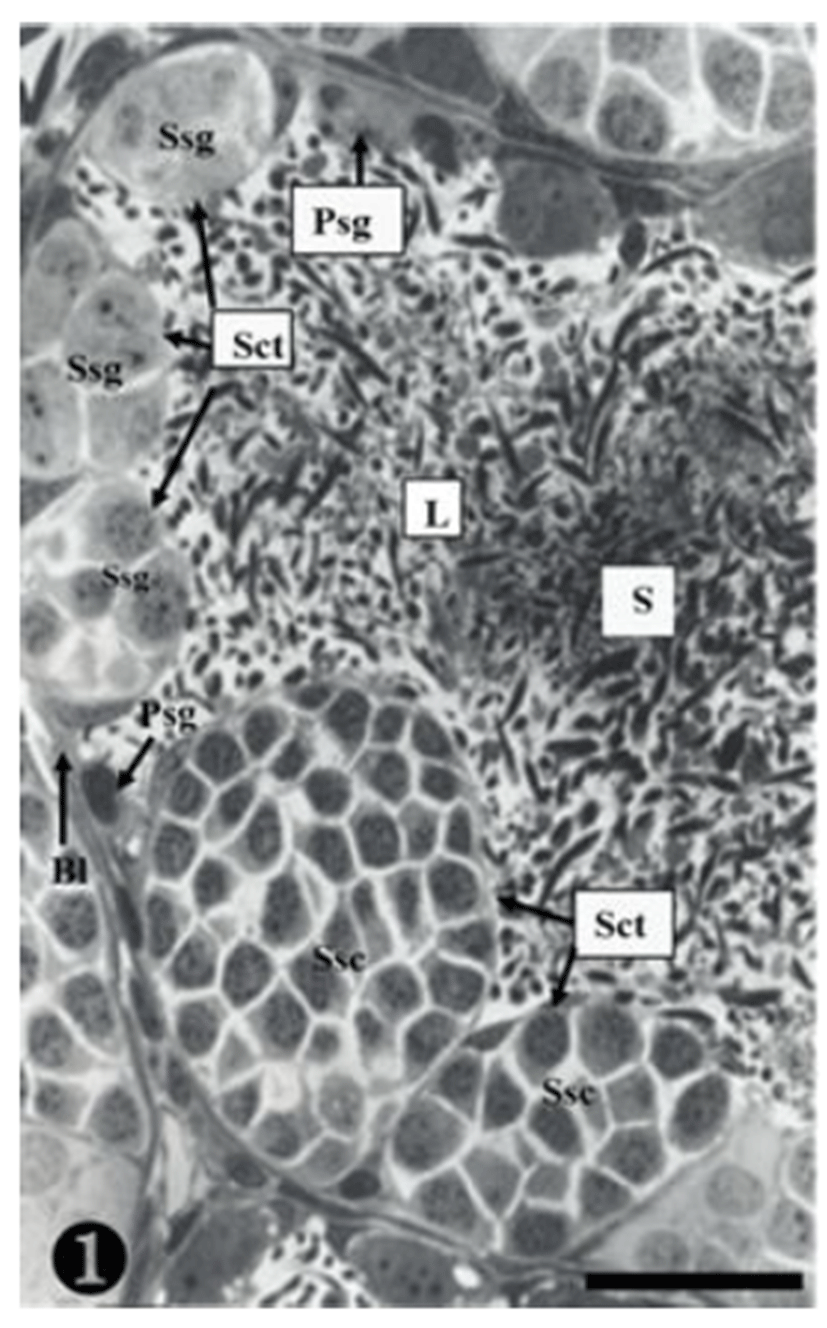
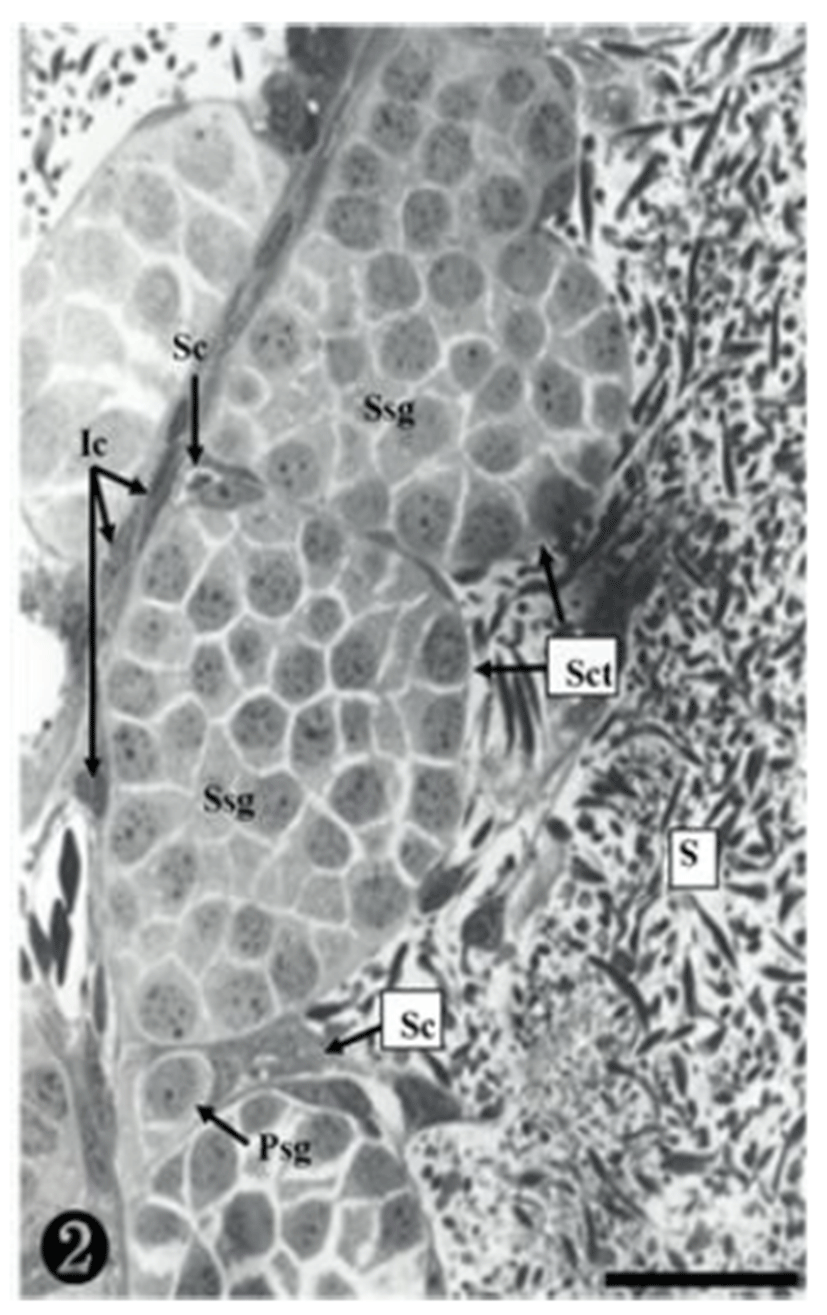
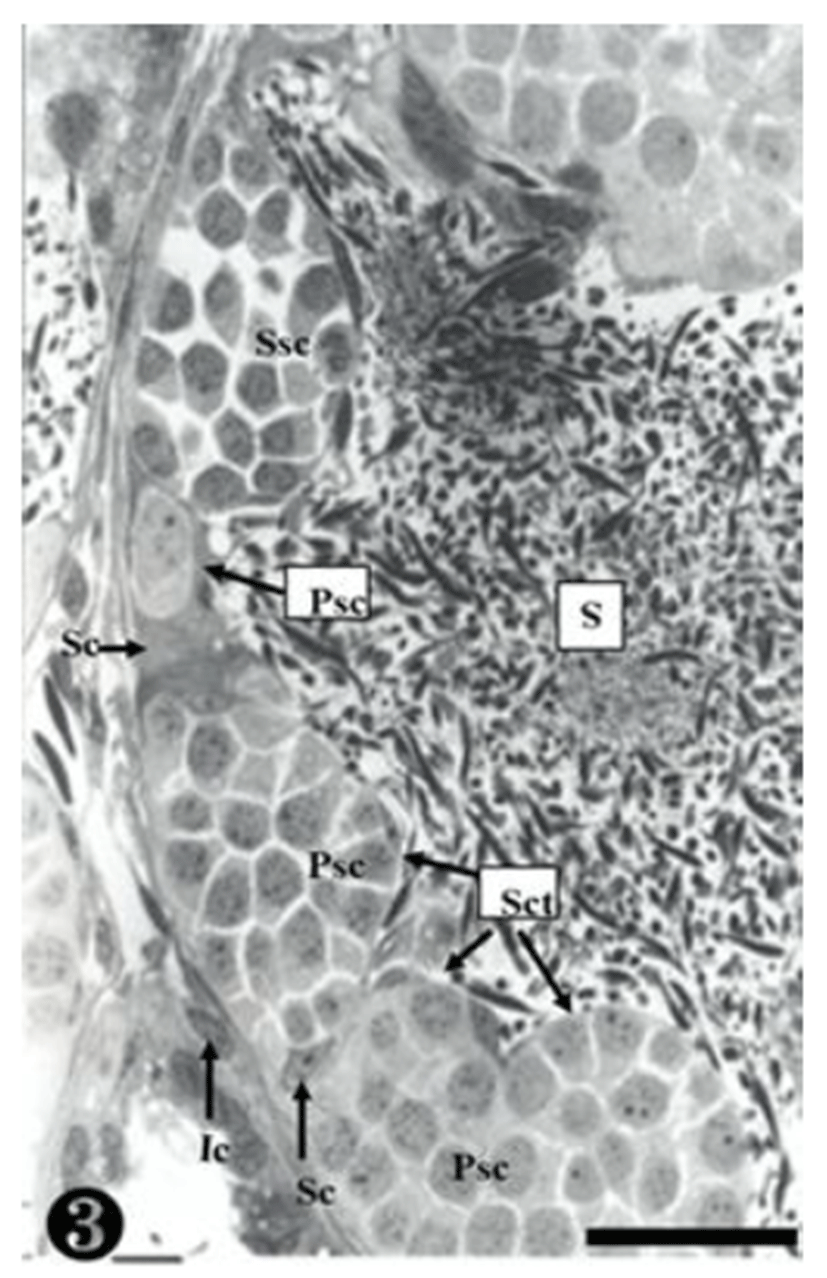
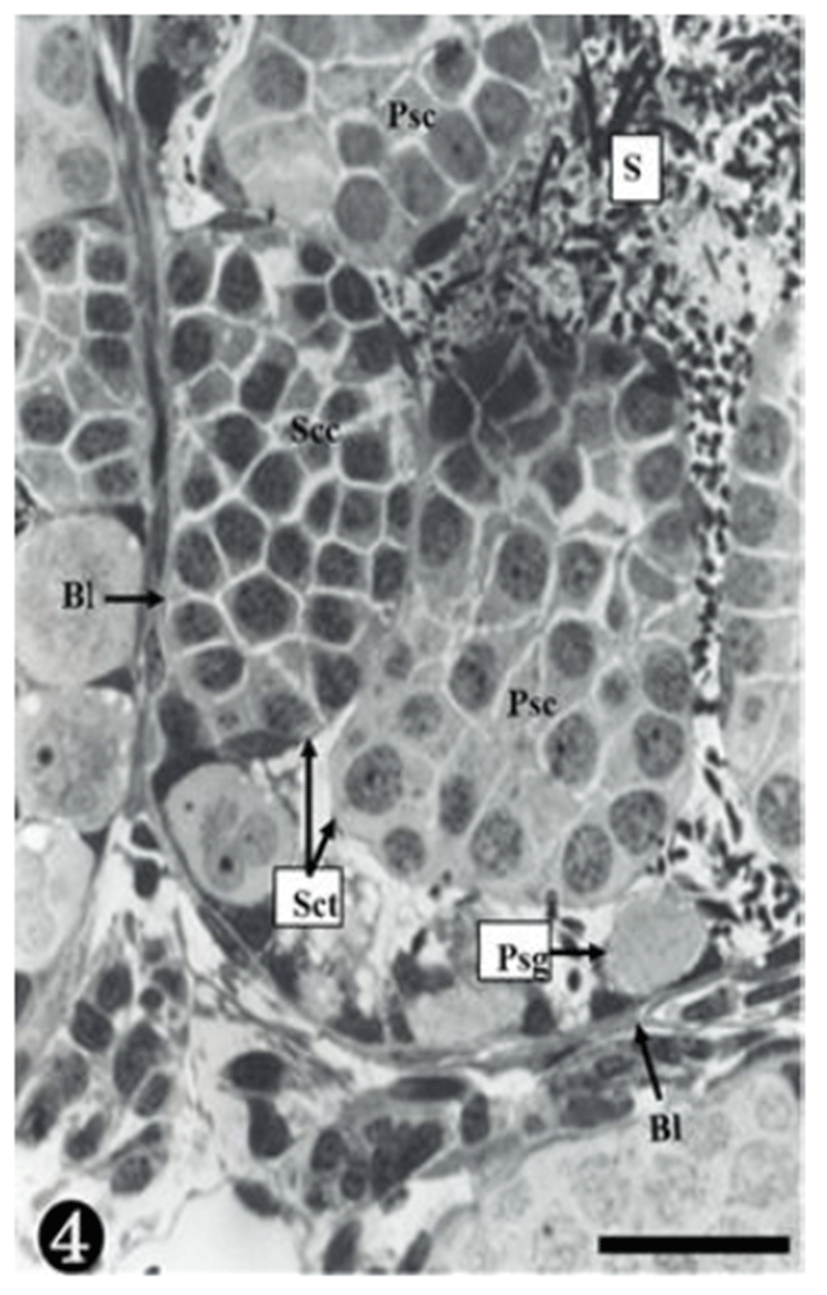
Both primary spermatogonia (Psg) and secondary spermatogonia (Ssg) were located near the seminiferous basal lamina (Bl), and numerous sperm (S) were observed in the lumen (L) of the seminiferous tubule. The primary sper-matogonia in this cycle did not form spermatocysts (Sct) (Fig. 1).
Many secondary spermatogonia (Ssg) were found in spermatocysts (Sct), around which many Sertoil cells (Sc) were found. Numerous spermatids were located around the spermatocyst (Sct) and the lumen (L), and the interstitial cells (Ic) between the seminiferous tubules were observed (Fig. 2).
The primary spermatogonia (Psg) were spread near the basal lamina (Bl) and many primary spermatocytes (Psc) were found in the spermatocyst (Sct). These spermatocytes showed somewhat different concentrations of nucleoplasm, due to the differences of serial cellular division. The cellular size of the secondary spermatocytes (Ssc) in the spermatocyst was smaller than that of the primary sper-matocytes, but their chromatin was much thicker, whereas the nucleus was smaller. Tremendous amounts of sperms (S) were observed near the spermatocyst (Fig. 3).
As shown in the above stages, the primary spermatogonia were located near the basal lamina, and primary and secondary spermatocytes were observed in the spermatocyst. Spermatocysts where numerous primary spermatogonia were observed were located in the inner space adjacency far away from the basal lamina. The nucleoplasm was much more concentrated than in stage III. The size of the secondary spermatocytes was smaller than that of the primary spermatocytes, In addition, their chromatin was much thicker, whereas the nucleus was smaller (Fig. 4).
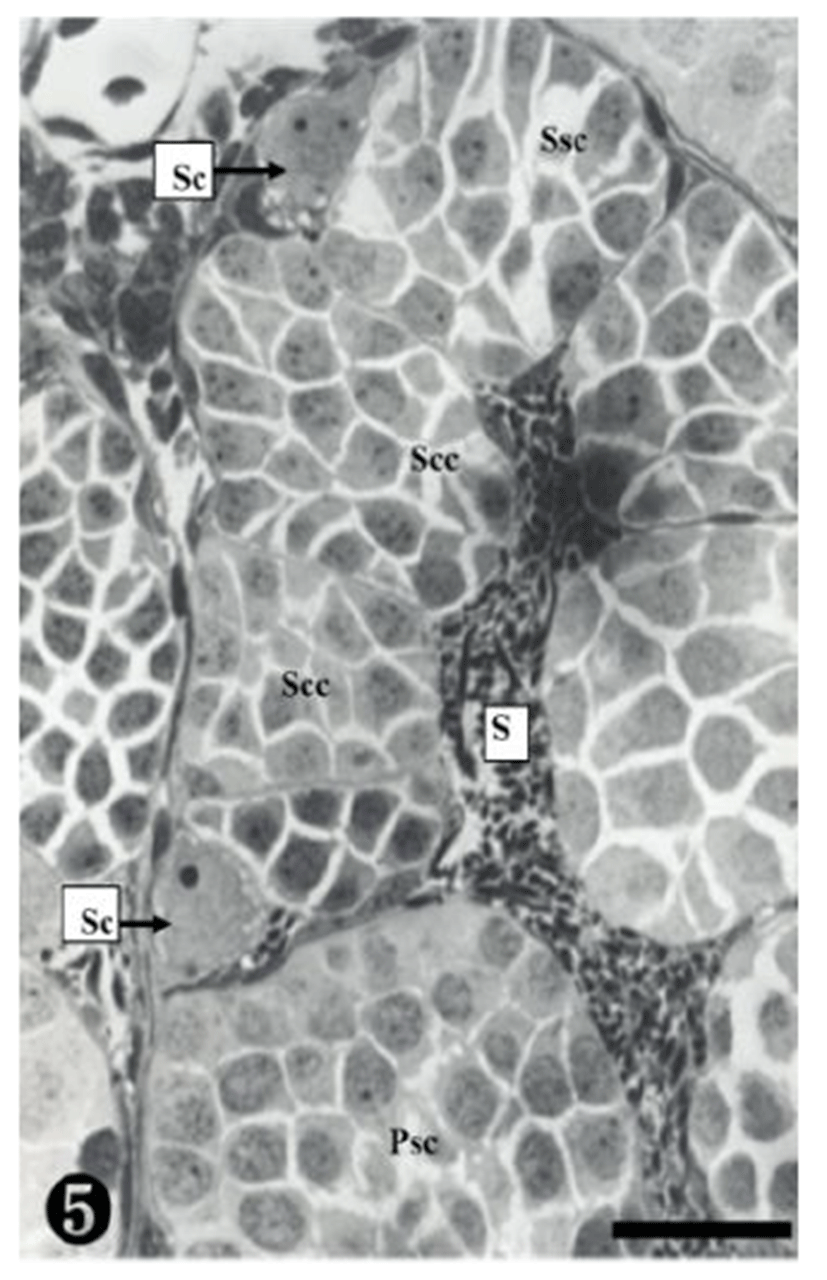
A group of secondary spermatocytes was created, located in the spermatocyst. In addition, Sertoli cells were observed around the spermatocysts (Fig. 5).
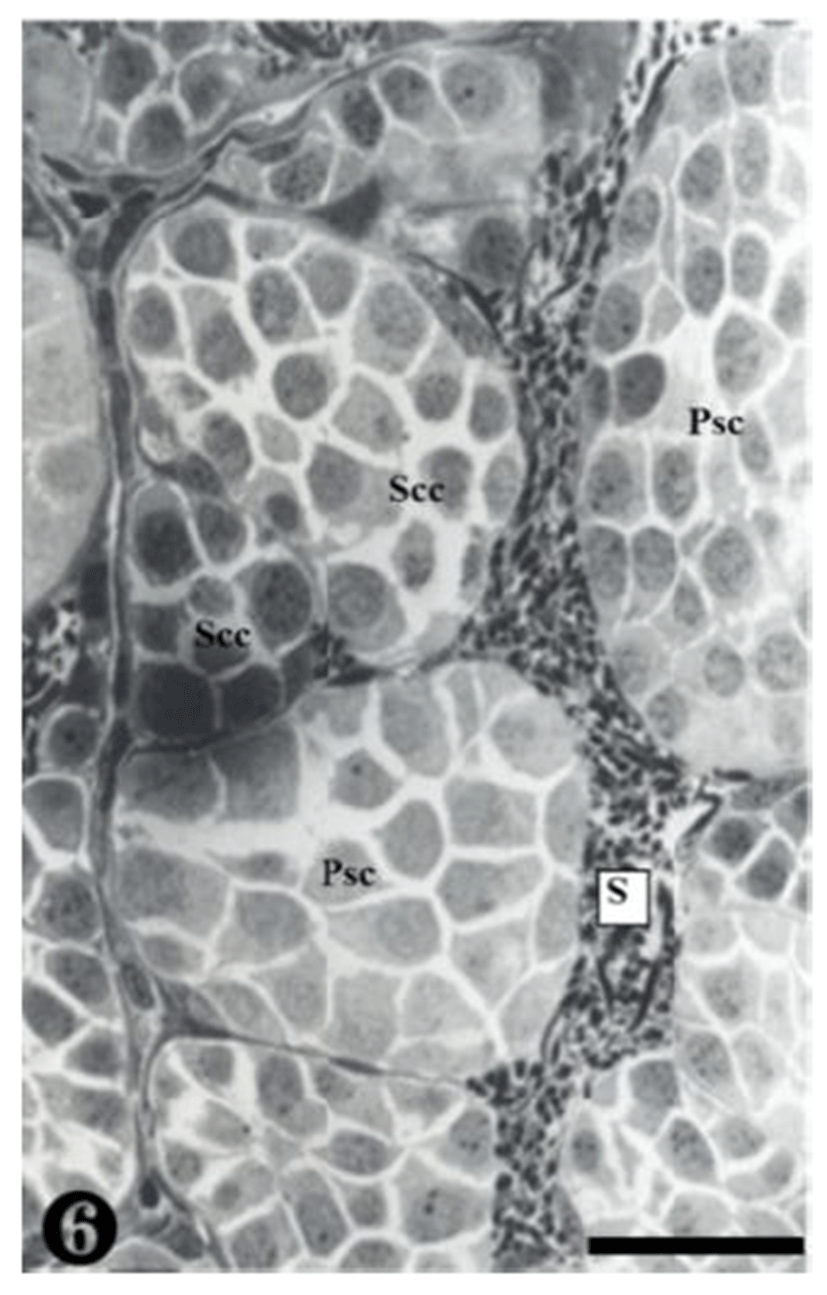
Secondary spermatocytes were observed in the spermatocys, and the spermatocysts composed of primary spermatocytes showed different concentrations of nucleoplasm due to differrences in the serial cellular division (Fig. 6).
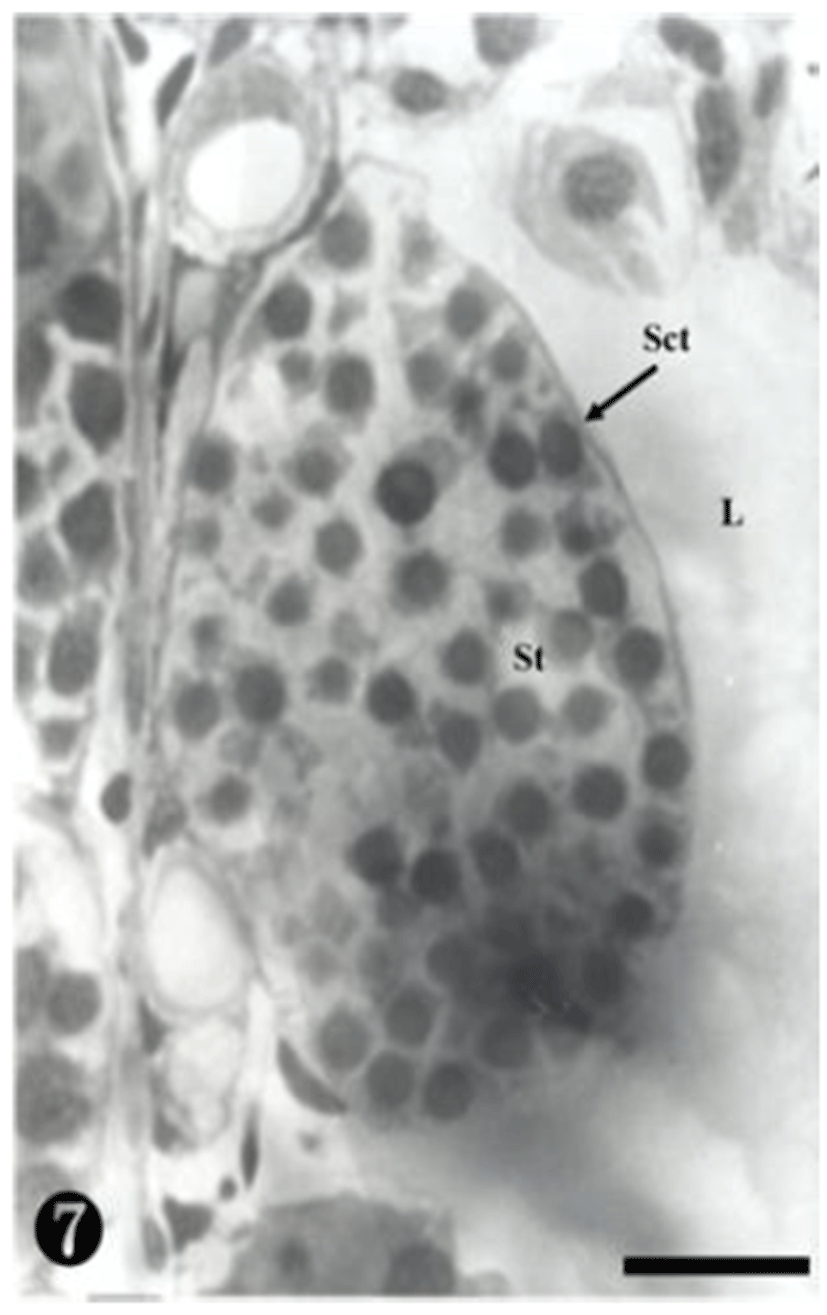
At this stage, the secondary spermatocytes were much more divided, creating spermatids in the maturing process. They were also found in the spermatocyst (Fig. 7).
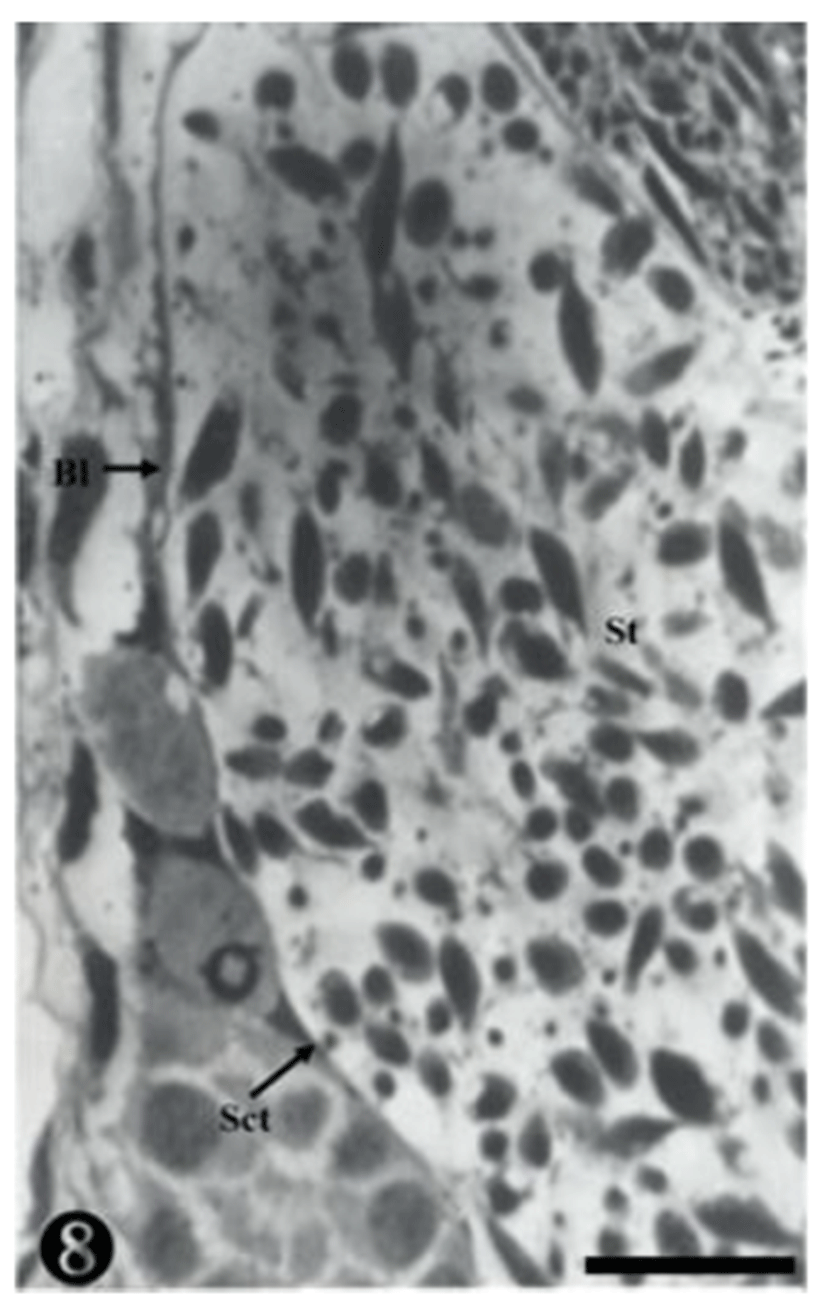
The nucleoplasm of the sperm cells near the basal lamina was observed to be much more concentrated, and some matured sperm cells were also observed. They were still located in the spermatocyst (Fig. 8).
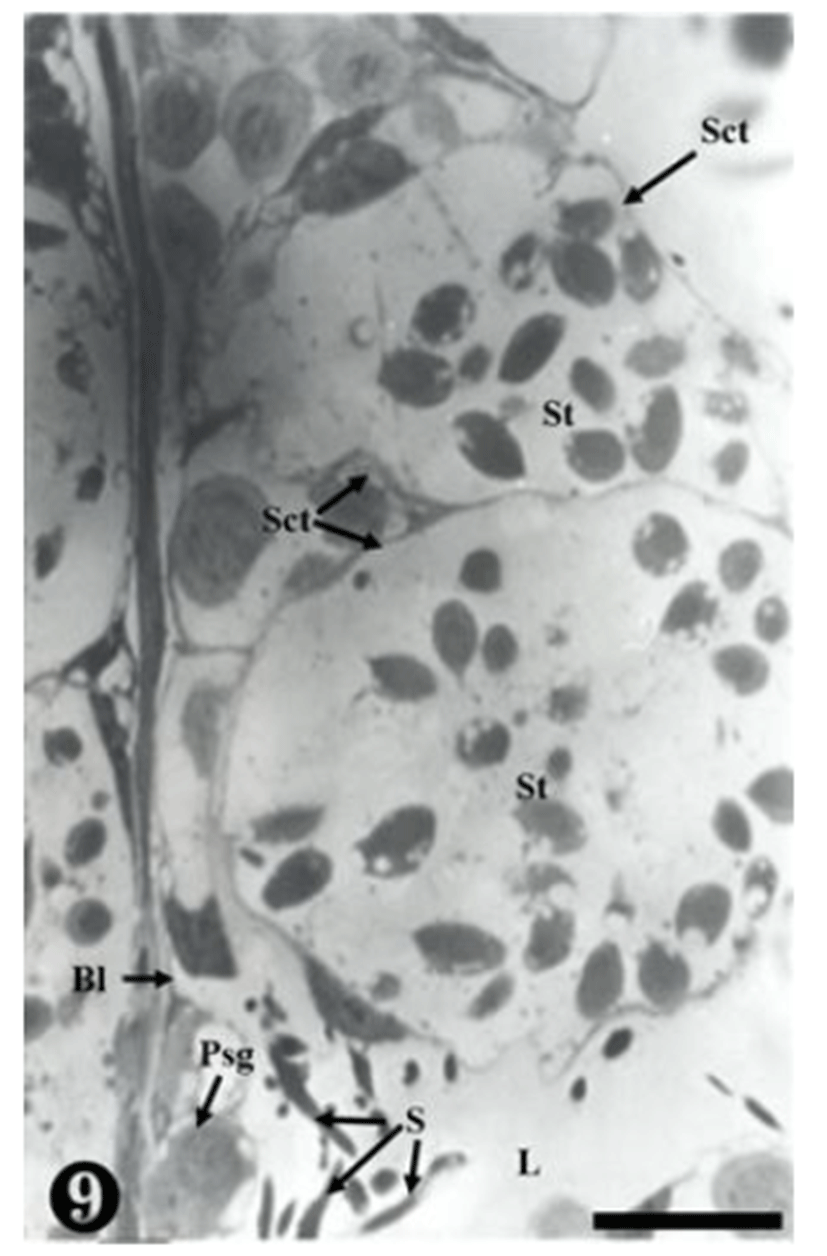
The primary spermatogonia were located in the basal lamina, whereas the matured sperm were detached, remaining in the inner seminiferous tubule. Some sperm were observed in small and large spermatocysts, while some were observed on the wall-side of the spermatocyst (Fig. 9).
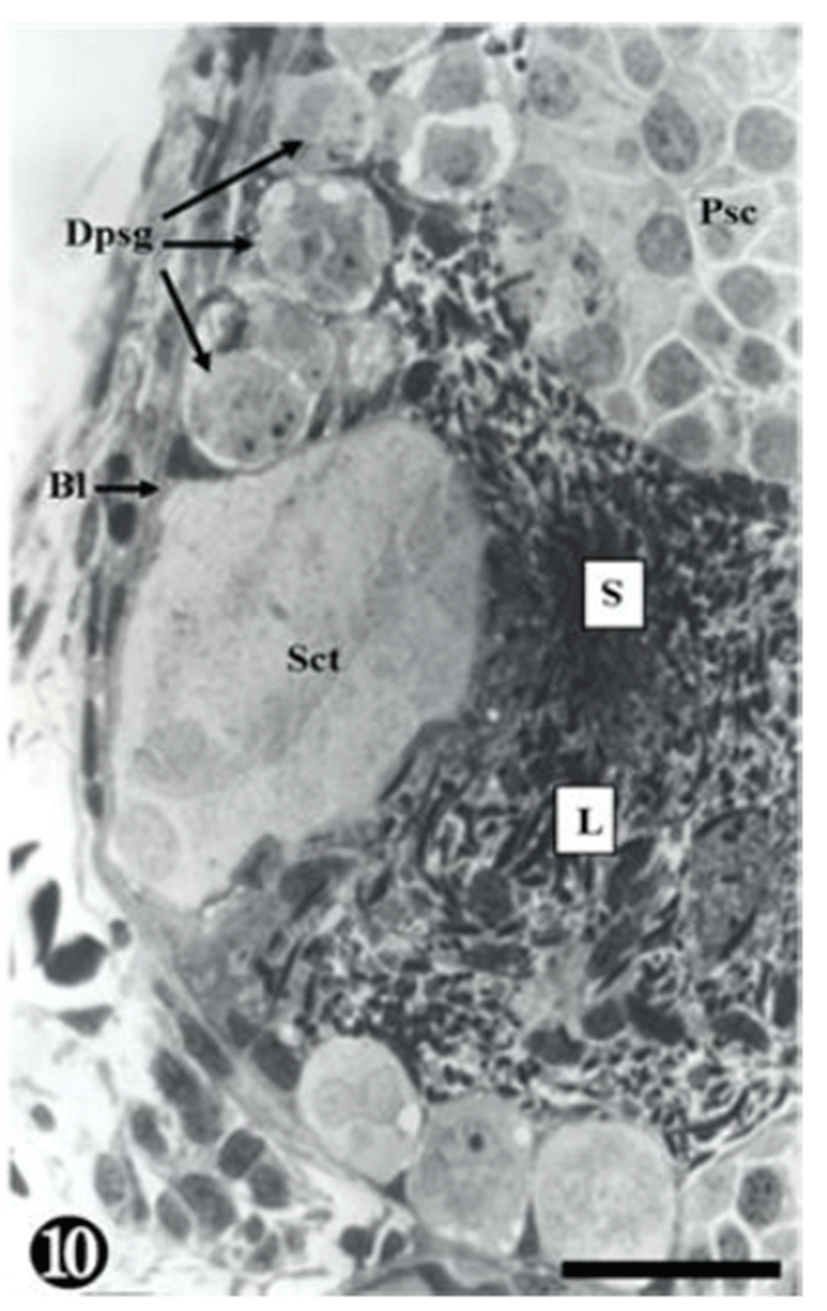
Degenerating spermatogonia (Dpsg) were found near the basal lamina, whereas the matured sperms were detached; therefore, the cyst was found to be almost empty. The inner seminiferous tubules were filled with countless sperm (Fig. 10).
DISCUSSION
Amphibian spermatogenesis includes germ cell cysts and seminiferous lobules. Meiosis is started by the division of the primary spermatocytes. In this step of the sperm formation process, the reproductive cells divided from the primary spermatogonia in the seminiferous tubule create a single cyst once they become secondary spermatogonia. Each germ cell cyst is filled with reproductive cells at the same division stage, which are to be divided simul-taneously within the cyst. The division of reproductive cells, therefore, depends on the environmental conditions in the habitat (van Tienhoven, 1983).
In this study, the division of reproductive cells in Bombina orientalis was divided into primary spermatogonia, secondary spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids and sperm, just like the stages proposed by Burgos & Fawcett (1956). In addition, the seminiferous epithelium cycle was divided into a total of 10 stages, according to the cellular maturation process and the morphological characteristics. The nucleoplasm became more concentrated from the primary spermatocytes to the secondary spermatocytes, while the size of the cell became smaller. In contrast, the nucleoplasm of the maturing germ cell became more concentrated than the germ cell at the earlier stages, whereas the nucleus and cytoplasm grew more. The spermatocysts were not created in the primary spermatogonia, but was directly attached to the semini-ferous lobule, being independent from other germ cell cysts. Spermatids were created from the secondary spermatocytes and remained in the spermatocyst, from which they later emerged after becoming fully matured. In other words, spermiation occurred and the cyst, composed of mature sperm, was disassembled, discharging into the inner seminiferous lobule (Pierantoni et al., 2002).
The seminiferous tubule lumen were not created, and the process was explained by 4 stages: the seminiferous tubule was created in the early sperm formation stage (Stage I) where only small sperms remained; the lumen of the seminiferous tubule were created and numerous sperms remained but did not move to the lumen in the later sperm formation stage (Stage II); the matured sperms moved to the seminiferous tubule lumen (Stage III); and the sperms and primary spermatogonia remained in the seminiferous tubules after the discharge of sperm (Stage IV) (Nagahama, 1986). In contrast, Bombina orientalis underwent maturation in the spermatocyst, from which the matured sperms fell out (Ko, 1997a, 1997b; Shin & Ko, 2014). This study presented the same results. However, R. catosbeiana showed an 8-stage seminiferous epithelium cycle, which is 2-stages faster than Bombina orientalis (Fig. 1). In other words, like B. orientalis, the sperm of R. catosbeiana matured in the spermatocyst without creating seminiferous tubule lumen, and then the mature sperms fell out (Go & Lee, 2001). Emergence of the matured sperms from the spermatocyst to the lumen was caused by degradation of the epithelial cyst surrounding the germ cell cyst, whereas the epithelial lining of the seminiferous lobule was located near the germ cell cyst at various division stages. Each cell cyst was filled with germ cells at the same division stage, therefore, division in the cyst occurred concurrently. This aspect is considered a special trait of Amphibians that cannot be found in mammals.
The spermatocysts were formed by the protrusion of Sertoli cells, according to Sprando & Russell (1988), whereas Burgos & Fawcett (1956) claimed that they were formed by the follicular cells at the division stage of germ cells. Burgos & Fawcett (1956) claimed that Bufo arenarum, the toad, differing from other frog classes, formed the spermatocyst at the primary spermatogonia. Considering the previous studies and the results of this study, focused on Bombina orientalis, it seems that spermatocysts composed of germ cells at the same division stage are formed, which show differences in the spermatocyte according to the species under the same class, even if the formation period of the spermatocyst is the same.

