INTRODUCTION
The maturation and reproduction in fish are regulating by neuropeptide and sexual hormone in reproductive endocrine system of the BPG axis. This axis is effected on environmental factor, mainly photoperiod and water temperature (Migaud et al., 2010). The Kisspeptin2 (Kiss2) is stimulate the secretion of hypothalamic GnRH neurons and GnRH neurons are regulates the secretion of pituitary GtH (Roa et al., 2011). The gonadotropins, FSH and LH induces the synthesis of sex steroids in the gonad and activate gonadal development and maturation (Tena-Semperea, 2010; Mechaly et al., 2013). Interaction between reproduction and growth occurs in many vertebrates and is particularly closely linked during sexual maturation in fish (Le Gac et al., 1993). GH is synthesized in the somatotroph and gona-dotrophs of pituitary gland. It is related to the somatic growth, reproduction, immunity, osmotic and ionic regulation in fish (Björnsson, 1997; Moriyama et al., 2000). The biological actions of GH are not restricted to growth promotion; instead they include gonadal development, appetite, and social behavior. GH system in endocrine network is highly intricate, including many environmental factors appropriate for the diverse physiological circumstances in which GH is involved.
Generally, many fish are responsive to changes in photo-period with alterations of growth rate and synchronizers of seasonal reproduction, which is generally directly related to day-length (Boeuf & Le Bail, 1999; Falcon et al., 2010). Long photoperiods have been demonstrated to increase the growth rates of salmonids independent of temperature (Villarreal et al., 1988; Saunders et al., 1989; Leiner & Mackenzie, 2001). These variations in growth and repro-ductive events suggest that light influences the secretion of brain-pituitary system in fish endocrine, such as growth hormone, Kisspeptin, GnRHs, and GtHs.
Olive flounder, Paralichthys olivaceus is economically important aquaculture fish in Korea. Although it is well established that maturation and spawning of olive flounder is induced by photoperiod manipulation (Min, 1988; Hur, 1991; Kim et al., 2013), the physiological mechanism in endocrine system is insufficient. Therefore, the present study investigated possible involvement of photoperiodic regulation on brain-pituitary axis in endocrine system of female olive flounder. In addition, real time qRT-PCR analysis compared expression level of Kiss2 and sbGnRH mRNA in hypothalamus and FSH-β, LH-β and GH mRNA in pituitary before and after spawning.
MATERIALS AND METHODS
Olive flounder (total length 37-39 cm, body weight 580-600 g) being reared at Hanchang Fishery Co. Ltd., located in Seogwipo, Jeju, South Korea, were examined. Based on previous study (Kim et al., 2013), the experimental fish were divided into two groups; the control group under a condition with natural photoperiod and the treatment group under an artificial condition with controlled photoperiod (15L: 9D= light on 0600h). They were reared for approxi-mately 11 months from August 2013 to June 2014. Seven to ten fish from each group were randomly selected to compare ovarian development and Kiss2, sbGnRH, GtHs and GH expression under different conditions for three times; one in December 2013 (Growth period), another in March 2014 (Spawning period), and the third in June 2014 (Spent period). The experimental fish were anesthetized with 2-phenoxyethanol (Sigma-Aldrich, St. Louis, Mo), then hypothalamus, pituitary and ovary were extracted. The extracted ovary of each specimen were weighted for calculation of the gonadosomatic index (GSI=gonad weight/ body weight×100). For the histological analysis, ovaries samples were fixed in Bouin’s solution, embedded in paraffin, sectioned 5 μm thickness and stained with haematoxylineosin. The hypothalamus and pituitary sample were kept at –80°C until total RNA extraction.
The total amount of total RNA from the hypothalamus and pituitary samples was extracted using RNAiso Reagent (TaKaRa Bio, Kyoto, Japan). The total RNA was treated with RQ RNase-free DNase (Promega, Madison, WI) to prevent genomic DNA contamination. The hypothalamus and purity of the total extracted RNA from the pituitary was examined with the ratio of 1.87 and 2.01 for A260/ A280 ratio. From 1 μg of the extracted total RNA, the reverse transcription reaction was performed to synthesize cDNA using the Transcriptor First strand cDNA Synthesis kit (Roche Diagnostics, Mannheim, Germany).
Primers for the experiments were produced by Kiss2, sbGnRH, FSH-β, LH-β, and GH genes of P. olivaceus that are listed on NCBI databse (Table 1). A qPCR was con-ducted with 20 ng of cDNA using SYBR green premix PCR kit (TaKaRa-Bio) in CFX96™ Real-time System (Bio-Rad, Hercules, CA). PCR was performed at 95°C after the initial denaturation. Afterwards, the PCR reaction was per-formed by 40 cycle of denaturation for 45 s at 94°C, annealing for 45 s at 58°C, and extension for 1 min at 72°C. Expression of the Kiss2, sbGnRH, FSH-β, LH-β and GH genes in each sample was normalized to the amount of the internal control EF1-α gene.
RESULTS
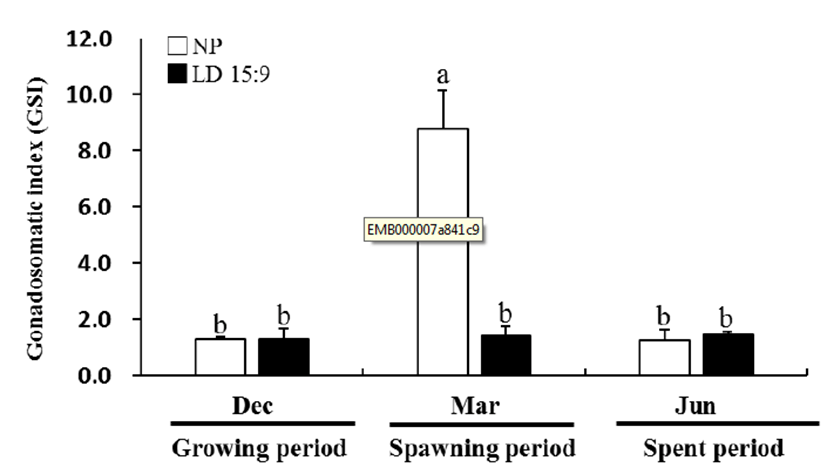
In December 2013, the GSI of natural condition and long photoperiod group was 1.29 ± 0.10 and 1.31 ± 0.15, respectively, showed no significant statistical difference. However, in March 2014, GSI of natural condition showed drastically increased at 8.79 ± 1.36, showing a significantly difference (P<0.05) (Fig. 1).
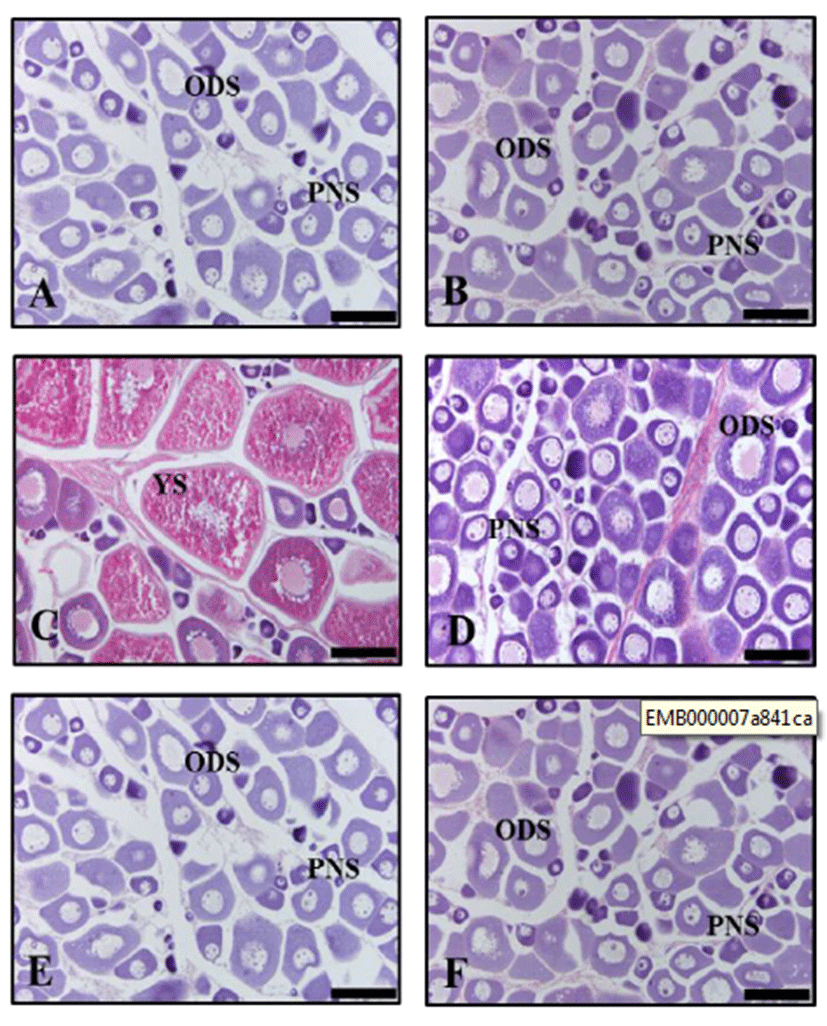
In December 2013, the ovaries of natural condition and long photoperiod group were growing phase, mainly con-tained oil-droplet stage oocytes of 80 to 150 μm in diameter (Fig. 2A, B). In March 2014, the ovary of natural condition became mature as vitellogenic oocytes of 220 to 500 μm in diameter (Fig. 2C). But the ovary of long photoperiod group were immature, mainly contained oil-droplet stage oocytes (Fig. 2D). In June 2014, the ovary of natural condition were spent phase, mainly contained peri-nucleolus stage oocytes and oil-droplet stage oocytes (Fig. 2E). The ovary of long photoperiod group were still growing phase, contained the oil-droplet stage oocytes (Fig. 2F).
In the natural condition, the expression levels of Kiss2 mRNA was significantly decreased at March 2014 than at December 2013 and June 2014. However, in the long photoperiod group, the expression levels of Kiss2 mRNA had no significant statistical difference during experimental periods (Fig. 3A). In the expression pattern of Kiss2 mRNA of natural condition, non-significant increase of Kiss2 was observed between growth period and spent period, but its expression declined significantly during spawning period (p<0.05).
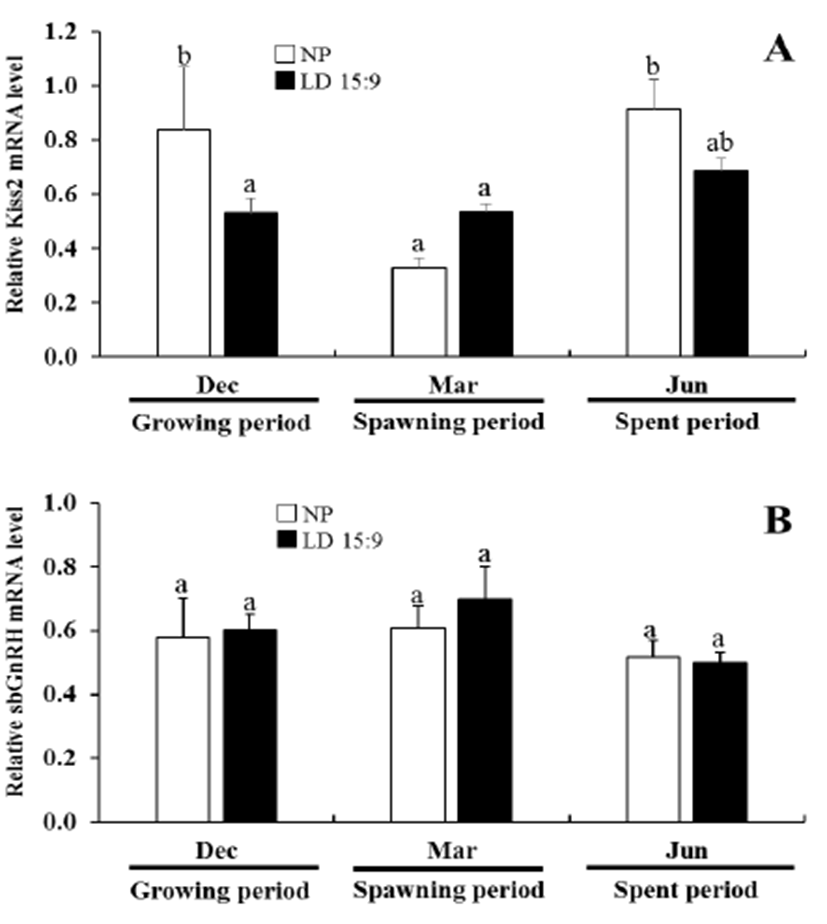
The expression levels of sbGnRH mRNA of natural condition and long photoperiod group had no significantly difference throughout during the experiments (Fig. 3B).
In the natural condition, the expression FSH-β mRNA at the December showed lower levels. However, the March experiment showed drastically increased levels of FSH-β mRNA determining significantly difference (P<0.05). In June 2014, the FSH-β mRNA expression were declined and reached low levels. In the long photoperiod group, the expression of FSH-β mRNA at the December showed higher levels. And, FSH-β mRNA expression level slightly declined from March to June, but the changes were not significant (Fig. 4A).
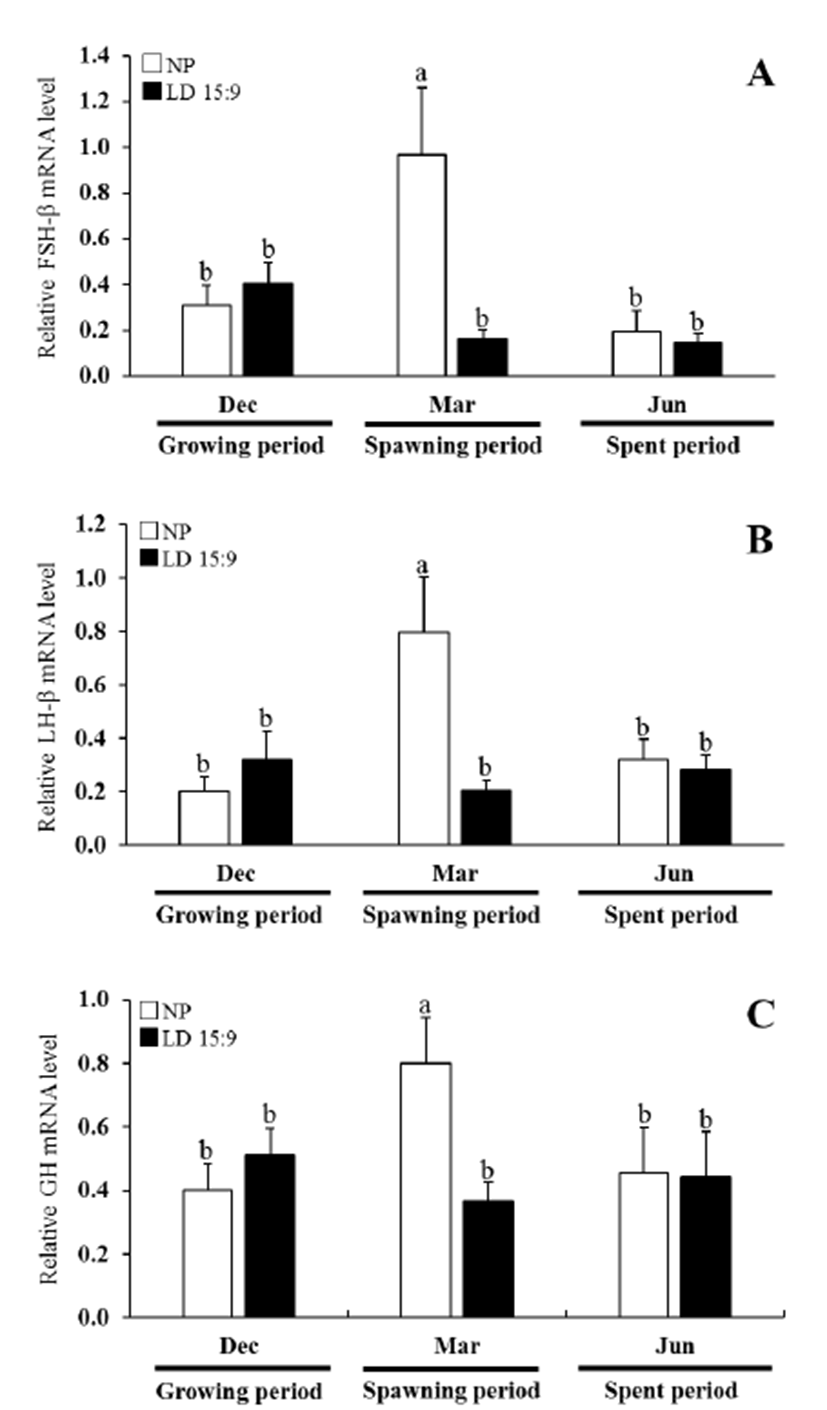
The LH-β mRNA expression profile showed a similar pattern to that of FSH-β mRNA expression levels. In the natural condition, the LH-β mRNA showed lower levels. During March, LH-β mRNA expression increased signifycantly and reached higher levels (p<0.05). In the long photoperiod group, the LH-β mRNA showed lower levels throughout all of the experiments (Fig. 4B).
In the expression pattern GH mRNA of natural con-dition, the GH mRNA level drastically increased at March. In particular, levels were approximately 2-fold higher than long photoperiod group. In the long photoperiod group, the expression of GH mRNA at the December showed higher levels. And, GH mRNA expression level slightly declined from March to June, but the changes were not significant (Fig. 4C).
DISCUSSION
In the present study, we investigated effects of artificial photoperiod manipulation on maturation of female olive flounder by reproductive endocrine system of the BPG axis. Firstly, we investigated effect ovarian development by long photoperiod manipulation using histological analyses, and compared expression level of Kiss2 and sbGnRH mRNA in hypothalamus and FSH-β, LH-β and GH mRNA in pituitary before and after spawning.
In fish, gonadal maturation and reproduction is regulated by a complex network of BPG axis. This axis is effected on environmental factor, mainly photoperiod and water temperature (Migaud et al., 2010). In Eurasian perch, 24-h photophase inhibit the initiation of gametogenesis and plasma testosterone and estradiol-17β levels were lower than in fish from the natural treatment. In particular, water temperature is not the only essential factor in the repro-duction timing of this species (Migaud et al., 2004). Gilthead seabream, Sparus aurata, that spawns in the winter, also showed low GSI when treated under long photoperiod of 15.5L:8.5D until the spawning season (Kissil et al., 2001). In a previous study, we confirmed that long photoperiodic conditions regulates sex maturation of olive flounder (Kim et al., 2013). In this study, continuous long photoperiod (15L:9D) treatment from August (post-spawning phase) was inhibited gonadal development of female olive flounder.
The external cues stimulate hypothalamic neuropeptide of the brain and the neuropeptide as a kisspeptin and GnRH are regulates the secretion of pituitary GtH (FSH and LH), which in turn activate gonadal development and maturation (Tena-Semperea, 2010; Mechaly et al., 2013). The two kisspeptin genes in fish have revealed the presence of Kiss1 and Kiss2 (Felip et al., 2009). These genes are potential regulators of reproduction and stimulates the release of GnRH (Kitahashi et al., 2009) and regulated by environmental factors such as photoperiods, steroid hormones, metabolic signals and stress (Parhar et al., 2012). In the chub mackerel, during late spermatogenesis and early vitellogenesis, Kiss2 levels increased slightly, followed by a significant decline during spermiation or late vitellogenesis in males or females, respectively (Selvaraj et al., 2010). The Kiss1 receptor mRNA expression was lower during advanced stages of spermatogenesis in the fathead minnow (Filby et al., 2008) and oogenesis in the grey mullet (Nocillado et al., 2007). In this study, the Kiss2 expression level a significant declined in March (spawning period), and GtH level significantly increased at this season in natural photoperiod group. However, in long photoperiod group, the Kiss2 and GtH expression level had no significant statistical difference throughout all of the experiment.
The somatogenic and gonadotropic axes have been known to be closely linked during growth and sexual maturation in fish. GH is produced and secreted anterior pituitary, is regulated by neuroendocrine factors from the hypothalamus. GH is directly regulated somatic growth (Moriyama et al., 2000) and it’s related to the other physiological functions of fish such as reproduction, immunity, osmotic and ionic regulation (Björnsson, 1997). In hypophysectomized male and female killifish, treatment with recombinant salmon GH prevents gonadal regression and stimulates testosterone and estradiol production (Singh et al., 1988). In the mecha-nism of GH stimulatory action on steroidogenesis using ovarian tissue of spotted seatrout, GH is stimulated ovarian aromatase activity (Singh & Thomas, 1993). GH can potentiate GtH-II stimulated steroidogenesis (Trudeau, 1997). In this study, FSH-β, LH-β and GH mRNA expression levels increased during spawning period in natural photoperiod group. However, in the long photoperiod group, FSH-β, LH-β and GH mRNA expression levels did not show any significant fluctuation.
In conclusion, artificially exposed long photoperiod regulated kiss2 mRNA in the hypothalamus during spawning periods. Further, FSH-β, LH-β and GH mRNA expression levels in pituitary were found to fluctuate during spawning season. These results suggest that expression of the neuro-Kisspeptin in the hypothalamus, GtH and GH in the pituitary would change in response to photoperiod and their possible involvement of photoperiodic regulation in reproductive endocrine system of the BPG axis.







