INTRODUCTION
As a one of cryopreservation methods, vitrification that does not require any expensive equipment and long working hours is globally used for preservation of human embryos in fertility clinic (Loutradi et al., 2008; Youssry et al., 2008). This method can prevent potential damages in embryos by reducing ice crystallization (Rall & Fahy, 1985). The successful vitrification is influenced by various factors including the types of tools, conditions of freezing and thawing process, cryoprotectants, exposure time in equilibration solutions and storage methods (Vajta & Nagy, 2006).
In the past few years, many researchers have been studied about cryopreservation tools that are divide open-type and closed-type as a one of important factor. During the freezing process using open tool, embryos directly contact to liquid nitrogen (LN2) and this contact cause high freezing rate and reduction of chilling injury (Vajta & Kuwayama, 2006). However, potential risk of cross-contamination by direct contact between embryo and LN2 during cryopreservation had been reported (Bielanski et al., 2000, Vanderzwalmen et al., 2010). On the contrary, closed tools could prevent infection agents and disease transfer because it eliminates direct contact between the embryos and LN2. However, closed tools cause reduction of low freezing rate (Vajta et al., 2015, De Munck et al., 2016). Although there are still questionable and controversial about between open and closed tools, many oocytes and embryos in human are usually vitrified with open tools worldwide (Vajta et al., 2015).
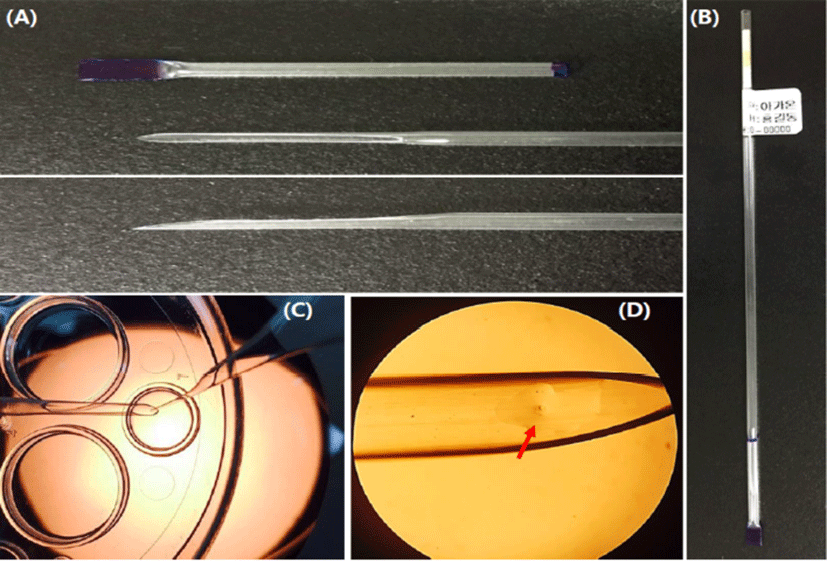
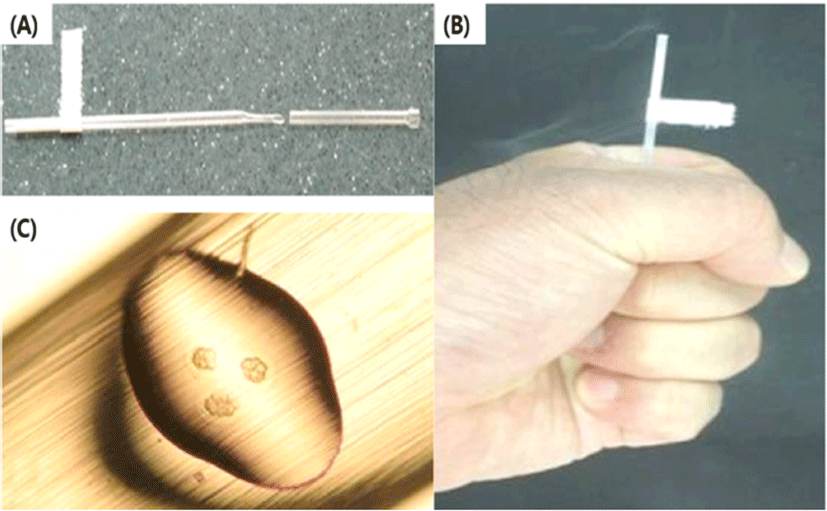
The modified cut standard straw (MCS) is one of handmade open tools. A few studies have recently been reported that MCS is used for vitrification of mouse oocytes, embryos and human embryos (Kim et al., 2010; Shin et al., 2011; Lim et al., 2013; Jung & Cheon, 2014). As a one of commercial tools for vitrification, MCS was directly immersed into the warming solution (WS) for increasing of warming rate (Vajta et al., 2015) and it easy to use. However, the existing MCS is difficult to immerse into the WS (Fig. 3), thereby embryologists grab the MCS for warming before dipping into WS.
Therefore, we modified the structure of the MCS to solve the inconvenience and this modified straw was called the ‘Long Cut Straw (LCS)’. This study was conducted to investigate whether the LCS could be useful as a stable tool for vitrified-warmed human blastocysts.
MATERIALS AND METHODS
A total of 138 vitrified-warmed cycles were used for investigation between November 2013 and November 2014 in this study. Women, who are ≥38 years old of age, are either poor responder and with husbands who have severe male factor and surgical retrieval sperm were excluded. This study was approved by the Agaon Fertility Clinic Institutional Review Board (IRB reference number Agaon IRB-15-003).
Ovarian stimulation was conducted with either a gonadotropin releasing hormone (GnRH) agonist long protocol or a GnRH antagonist (Merck Serono, Italy) protocol. Human chorionic gonadotropin (hCG, 10,000 IU, Merck Serono, Italy) was injected to the women 36 hours before ovum pick-up. Oocytes were collected via transvaginal ultrasoundguided with a 19-gauge needle (Dukwoo Medical, Korea).
Mature oocytes were inseminated with either conventional insemination or intracytoplasmic sperm injection (ICSI), depending on the state of sperm, oocyte and previous history of patient, etc. The outcome of fertilization was assessed in 17-19 hours after insemination, when the presence of two pronuclei was observed. Up to three zygotes were cultured in 30 μL of micro-drop with either one-step medium (Life Global®, Belgium) or sequential medium (Vitrolife, Sweden) under covering with paraffin oil (Vitrolife, Sweden) in the atmosphere of 6% CO2, 5% O2 and 95% humidity at 37°C.
The LCS is pulled at the end part of a 0.25 mL straw (total length: 13 cm, cutting part: 1.5 cm) by heating using an alcohol lamp and then cutting it gentle at an angle (Fig. 3). The end part of the sealed cap was marked blue to distinguish the part in the LN2 (Fig. 1). The LCS was sterilized using ethylene oxide and then used it after two weeks.
Surplus embryos were cultured, until day 6, to the blastocyst stage after embryo transfer (ET). The blastocysts graded 3- 6 were cryopreserved by Gardner and Schoolcraft’s blastocyst scoring system (Gardner et al., 2000). Ethylene glycol (EG, Sigma-Aldrich, USA) and dimethyl sulfoxide (DMSO, Sigma- Aldrich, USA) were used as the vitrification solution (VS) which acts as the permeable cryoprotectants. The artificial shrinkage was performed using 29-gauge needles before vitrification of balstocysts. For the vitrification procedure, one to two blastocysts were placed into the VS #1 (PBS (SAGE, USA) supplemented with 7.5% EG + 7.5% DMSO + 20% SSS (IrvineScientific, USA)) for 1 min at room temperature (RT). The blastocyst was then transferred into the VS #2 (PBS supplemented with 15% EG + 15% DMSO + 20% SSS + 0.5 M Sucrose) for 25-30 second at RT. The blastocyst was then loaded onto the end part of the LCS as described (Cryotop method) by M. Kuwayama et al. (2005b). The blastocyst around VS #2 was nearly removed using pipette to make minimum volume size (Fig. 4) and then the LCS was plunged into LN2 after putting a cap on.
For warming, the sealed cap of the LCS was removed using the forceps inside the LN2 and then directly immersed into the first 1 M sucrose solution for 1 min at 37°C. The blastocyst was transferred by sequential exposure into the second 0.5 M sucrose solution for 3 min and was transferred into 0.25 M sucrose solution for 2 min, and lastly into the washing medium (PBS supplemented with 20% SSS) for 5 min at RT. The survival of vitrified-warmed blastocyst (VWB) was assessed under a stereoscopic microscope (Olympus, Japan) around 18-20 hours after warming.
Only re-expanded blastocyst transfer was performed using an ET catheter (COOK® Medical, Australia). Endometrium was prepared for VWB transfer either by artificial cycle protocols with exogenous estrogens or by natural cycles. In artificial cycle protocols, progesterone in oil (50 mg/day) was administered on daily basis when the endometrial thickness reached 8 mm or more, and the thawed embryos were transferred 5 days later. In natural cycle, the endocrine preparation of the endometrium is achieved by endogenous estrogen production from a developing follicle. Timing of embryo transfer is determined by administering hCG for triggering ovulation. One or two re-expanded blastocysts were transferred into uterus after 6 days of progesterone treatment. The rates of implantation and clinical pregnancy were confirmed by monitoring of the gestational sac at 6 weeks of pregnancy. Ongoing pregnancy was defined as showing a positive heart-beat at ultrasound scan after 7 weeks of gestation. Live birth outcomes including number of babies were investigated.
RESULTS
A total of 138 vitrified-warmed cycles had altogether 294 blastocysts vitrified using the LCS method. The mean age was 32.3 ± 2.5. A total of 294 blastocysts that were warmed, 294 blastocysts were recovered (100%), and 285 of those blastocyst were survived (96.9%).
ET was performed in all cases without cancellation. The mean number of transferred embryos was 2.1 ± 0.4. Mean implantation rate was 24.2%. The rate of clinical pregnancy was 36.2%, and 50 (36.2%) of those patients had an ongoing pregnancy with fetal heartbeat at 12 weeks of gestation. The rate of live birth was 31.2%. These results are presented in Table 1.
DISCUSSION
We described the simple warming method of the LCS as a useful and stable tool. LCS was directly immersed into the WS without pre-warming by performer’s hands and use of this tool showed the high recovery and survival rates for the VWB, followed by successful pregnancy and live birth. This study is first report regarding live birth after using the modified straw.
Kim et al. (2010) and Lim et al. (2013) reported that the survival rates of VWB using the MCS were 73.0% and 75.6%, respectively. On the other hand, our data showed higher survival rate of VWB (96.9%) using LCS than vitrified-warmed using MCS in other study. In addition, the results of present study showed a reasonable ongoing pregnancy (36.2%) and live birth (31.2%) rate of transferred VWB, which was vitrified-warmed by LCS, in human.
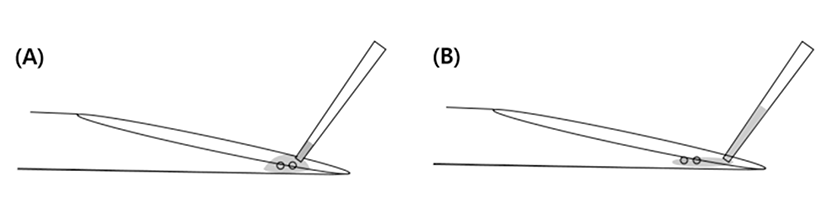
The end part of MCS have a narrow and short surface that caused occurrence of capillary action (Fig. 3C). Because of this structure, it is difficult to immerse into the WS. Therefore, the part of straw cap is removed using hands to grab it at performer’s hand temperature (Fig. 2B) Therefore, the end part of vitrified MCS is warmed using performer’s hand and then VWB are transferred using the pipette into the WS. During the pre-warming process, VWB are exposed to high concentration of cryoprotectant solution and it caused the risk of lost warmed-embryos. The chemical toxicity of cryoprotectant increased the potential risk of injury in vitrified human blastocyst (Mukaida et al., 2006). However, the LCS can solve these problems by elimination of unnecessary process of additional exposure to cryoprotectant.
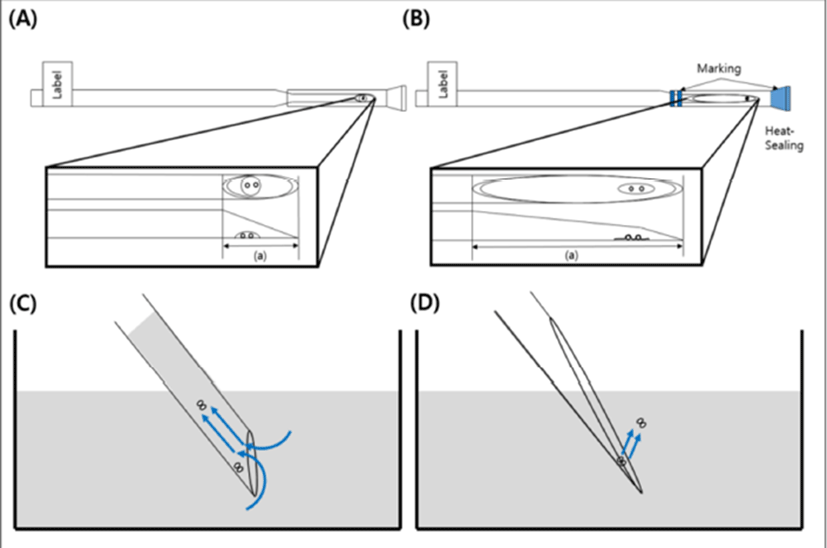
Previous study reported that the shape of in-house-made tools were difficult to make small volume of cryoprotectant for vitrification (Marco-Jiménez et al., 2016). However, the LCS is possible to load the minimum volume size (<0.1 μL) of cryoprotectant with blastocyst on the surface (Fig. 4B) because the end part of LCS with long length has a similar structure to the Cryotop as described by M. Kuwayama et al. (2005b). A lower volume of cryoprotectant during vitrification can reduced the rates of ice crystallization and chilling injury by increase of freezing rates (Cobo et al., 2008). Therefore, we expected that using the LCS might raise the speed of freezing and warming as commercial tools.
Some researchers have voiced out their concerns about the safety of the open tools for the freezing of sperm or embryo because there is a potential risk of cross-contamination (Bielanski et al., 2000; Bielanski et al., 2003; Bielanski & Vajta, 2009; Kuwayama et al., 2005a; Vanderzwalmen et al., 2010). As the LCS is an open tool, we cannot eliminate the potential risk of cross-contamination completely. However, the recent guideline of the American Society for Reproductive Medicine (2013) has stated this: “there also are theoretic infectious disease concerns with the use of open vitrification methods. However, infectious transmission has never been observed in reproductive tissues from this technique”. We thought that the guideline supports to resolve about a concern by the use of open tools. In addition, during the past few years, tens thousands of women had delivered healthy babies worldwide using the open tools (Vajta et al., 2015). Despite using the open tools for vitrification is a still questionable and controversial, this tools are still used to freeze oocytes and embryos worldwide.
In our center, the frozen embryos and sperm derived from any infected patients were separately stored from the frozen embryo and sperm derived from all other non-infected patients, using different containers. This is necessary to minimize any potential risk of cross-contamination.
The cost of the LCS is much lower than that of commercial kits (Gvakharia & Adamson, 2011). We expected that use of LCS, as a cost factor of cryopreservation, would lighten the financial burden of patients. In addition, the LCS is user-friendly and easy to use. We suggest that the LCS tool, which were used for vitrification of blastocyst stage, could provide us with acceptable clinical outcomes, live birth and has simplified the warming process. Further investigations on comparative studies regarding clinical outcome among various tools and larger data are needed.

