INTRODUCTION
Reproductive capacity of small mammals living in temperate region displays unique characteristics from species to species. Almost all the animals do not possess the reproductive function throughout the entire year (Choi & Han, 2010; Choi & Lee, 2012). This is a revolutionary consequence because a large amount of energy is required in maintaining the energetic sexual activity. Thus most of animals reproduce for a particular period of time in a year, sparing the energy and keeping the species unimpaired.
There are a number of reports, indicating that reproductive activities of mammals are altered by herbal extracts (Abdel-Rahman, et al., 1999; Tajuddin et al., 2004; Mishra & Singh, 2008; Yang et al., 2010; Lee, et al., 2013; Choi, et al., 2014). The bitter melon (Momordica charantia) is a tropical and subtropical vine of the family Cucurbitaceae, which is originated in India. it is grown in Asia, Africa, and the Caribbean regions, including Korea. The different parts of the plant have been used as a culinary materials of various foods in many countries. Also as herbal medicine, its extracts have been well-known for a hypoglycemic effect (Lo et al., 2013). Bitter melon reduces adiposity, lowers serum insulin, and normalizes glucose tolerance in rats fed a high fat diet (Chen et al., 2003). In addition, a variety of ailments are subjected to treat stomach, laxative, antibilious, emetic, anthelmintic agent, cancer, and infections (Wang et al., 2017). Some side effects are reported for diarrhea, abdominal pain, fever, and chest pain. But the symptoms are mild, requiring no further treatment.
Lately, there were some reports that the extract of the MC seeds caused infertility in male rat (Girini et al., 2005; Tumkiratiwong et al., 2014). In the examination of morphological changes in sperm of albino rats, the alcoholic extract of MC seed showed the disturbance in the plasma membrane as well as in the acrosomal membrane in scanning electron microscopy. The results indicate the anti-androgenic property of MC seeds (Girini et al., 2005). The antisteroidogenic activities as well as antisperma-to-genic effects was observed in male rat (Naseem et al., 1998).
The golden hamster is one of the seasonal breeders whose reproductive activities are active around summer season (Choi & Lee, 2012). In the winter climate the reproductive functions are completely arrested. This seasonal breeding strategy is determined by photoperiod that is the length of lighting in a day, which is surmised that the animal can predict possibly the annual cycle in a period of a year.
The fluctuating changes of seasonal reproductive function in the hamsters can be reproduced in the artificial lighting regime. When the reproductively mature male hamsters are transferred to SP (equal to and less than 12 hours of lights in a day), they lose generative activities, showing no functional spermatozoa in testes. If the length of lighting in a day is set to more than 12.5 hours (long photoperiod; LP) mimicking summer season, the generative functions are energetically resumed, doubtlessly promoted, and maintained afterward.
The goal of the present work was to investigate the effects of the MC extracts on the spermatogenesis in the testicular activity of male golden hamsters.
Materials and Methods
Seeds of Momordica charantia were purchased from the local traditional market in Korea. They were cultivated in designated area where the MC vine crept up the reticular-formed net supported by pillars. The riped yellow fruits were gathered and the skin with pulp inside the fruit was discarded. The seeds were collected by removing the red arils surrounding them. They were fully dried in shadow and ground by Osterizer blender. Seventy percent of alco-hol was used to immerse the powdered MC seeds for more than 60 days. The mixture was squeezed with woven texture to collect the fluid. The fluid was refined using coffee filters. Then it was dried in the oven kept at 60℃. The solidified product was milled to make fine powder. The yield of the extract was 5.9% w/w in terms of dried starting material weights. One gram of powder of the extract was corresponded to 16.9 grams of dried seeds of MC. The powered extract was light brownish. The extract was preserved in a freezer and was dissolved with drinking water prior to usage.
Adult male golden hamsters (Mesocricatus auratus) weighing between 140-150 g were used for the study. They were housed in plastic cages under LP conditions of light and dark (light of 14 h : darkness of 10 h) with an ambient temperature of 22±1℃. Reproductive activity of these hamsters are always active in the photoperiod. They were fed with standard laboratory mouse chow and tap water ad libitum. The condition of management of animals was approved by the Yongin University Institutional Ani-mal Care and Use Committee (YUIACUC-2015-04).
The golden hamsters were divided into four groups. The control animals were maintained in LP (light of 14 h : darkness of 10 h) or SP (light of 1 0h : darkness of 14 h) and received the vehicle (drinking water). The experimental animals were housed in LP. The MC extract was dissolved in vehicle and was administered orally for 56 consecutive days using an intubation needle. The animals were received the MC extract at doses of 1.5 g (high dose) or 0.3 g(low dose) per kg of body weights on a daily basis. During this experimental period the body weights were measured every week. In 9th week, the animals were mated with age-matching females who had experienced pregnancy. They were cohabited with females for 8 days. Then the male animals were sacrificed. At the end of experiments testes were isolated and weighed to compare between groups. The internal organs were also dissected and weighed. During the period of time, the behaviour of animals was observed to examine any abnormal phenomenon due to long-term treatment.
Male hamsters were housed together with female hamsters who had experienced pregnancy in advance to evaluate the effect of MC extract on the fertility. The estrous cycle of female golden hamsters are exactly 96 hours, which is 4 days. Eight days were allowed to make sure the mating. Males were then removed and sacrificed for the parameters of this experiment. The fertility capacity was determined in 3 full weeks. The number of offspring from females who gave birth were recorded.
Results
There was no any particular aberrant action in animals treated with the MC extract for 8 weeks. Thus, the MC extract was concluded not to induce any abnormal behavior.
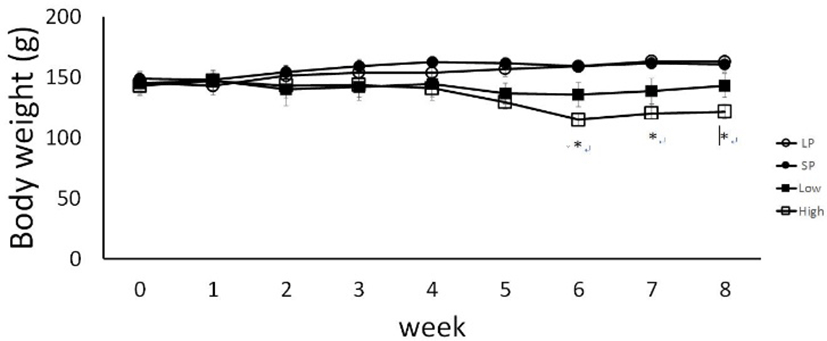
The body weights of hamsters were measured at the regular intervals to evaluate any abnormal aspects in growth (Fig. 1). The body weights of animals in both LP and SP control gradually increased from the beginning of the experiment. The change of body weights during the entire experimental period of time was matched with the growth pattern. On the other hand, the body weights were inclined to diminish in two weeks of MC extract administration compared to the LP and SP control animals. The gaps were distinctly enlarged on the fifth week of MC extract administration, culminated with statistical difference (P<0.05) on the sixth week, and later on slightly narrowed with similar significance at every week measured.
In order to examine any changes of internal organs, various organs were isolated and weighed at the end of the experiment (Table 1). Resultantly no weights of the organs were noticeably altered by the MC extract administration except for the reproductive-related organs, seminal vesicles and the epididymis. They were statistically (P<0.05) reduced in only SP animals.
LP: animals housed in LP and treated with vehicle, SP: animals housed in SP and treated with vehicle. Low and High: animals administered with low and high concentrations of the MC extract, respectively.
Data are represented as the mean±SEM (n≧3).
* Denotes statistically significant difference (p<0.05).
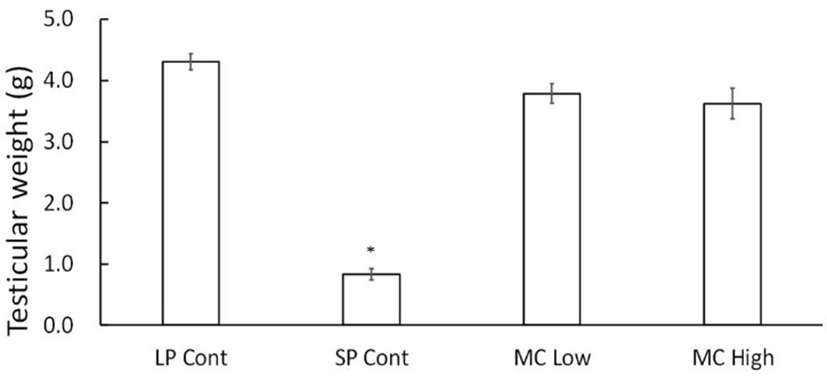
At the end of experiment the real weights of testes were in fact individually weighed. The changes of testicular weights are shown in Fig. 2. The mass of the testes was main tained in LP animals at the end of experiment but those in SP animals had statistically significant different (P<0.05) testes when compared to the testes of the rest. Both the low and high concentration of MC treatments showed same large testes as in the LP control animals. The low and high concentrations of MC treatments showed large testes, which means ineffectiveness in blocking effects on testicular mass that is normally represented by SP.
Some male hamsters mated with female hamsters who previously gave birth to litters begot offsprings in every group except the animals in SP group. The groups in which gave delivery demonstrated about same pregnant ratio as animals in LP control. The ratio of males who had delivery was around 60%, which is usual proportion of fertility fecundity in this golden hamsters. No statistically significant difference was observed in the weights of offsprings.
Discussion
MC is a herbal plant which is usually recommended for treatment of many diseases, particularly diabetes. However, some studies showed that despite the diverse beneficial effects of MC (Bai et al, 2016; Czompa et al., 2017), it has a detrimental role on the reproductive system of male rats (Tumkiratiwong et al., 2014). The results of the present investigation showed no significant effects on the reproductive capability that is governed by photoperiods in male golden hamsters.
As well-established, golden hamsters kept in LP have constant large mass of testes that implies sexually active functional activity (Choi & Han, 2010; Choi & Lee, 2012). But the exposure to SP induces the regression of testes in the animals within the period of time of 8 weeks as described previously. Photoperiods are absolute regulator of the reproductive activity.
One of the methods to evaluate toxicity of plant extracts is to measure any unnatural changes in the body weight. In the present investigation no alterations in the body weights were observed in both LP and SP control groups. But golden hamsters treated with MC extracts showed significantly (P<0.05) reduced body weights from the sixth weeks of the experiment, which is not the aim of the present examination. Nonetheless the extract of MC plant presents no general reproductive toxicity at the level of doses administered.
The present results showed that MC extract had the complete failure of blocking of fertilizing action of LP, which might be due to the possible shortage of the period of treatment time. But in other report, the authors reported reproductive toxicity effects in 6 weeks of treatment in rats (Tumkiratiwong et al., 2014). In case of golden hamsters, the period of 8 weeks has been reported to be enough to testify the sexual regression. With their results and the sufficient period of time of 8 weeks enough for induction of testicular regression in hamster species, the experimental period of time applied in this investigation is thought to elicit sexual involution. The possibility that longer administration could alter the photoperiodic effects on the sexual function in this animal is reasonably low.
Another point to be thought is a difference of animal species used in those experiments. The discrepancy could be due to the unique properties of animals, in which golden hamsters are seasonal breeders but rats are not. The extraction methods could be a factor induced the different results. The extraction method used in this experiment was nearly same as the previous reports in which the extraction agent was 70% ethanol (Tumkiratiwong, 2014). The concentration of the plant extract administered into the golden hamsters in this experiment was twice. The highest concentration administered into the animals here was 1.5 g per kg of body weight of animal, whereas Tumkiratiwong et al. used ethanolic seed extract at 0.8 g dry matter of per kg body weight. Nonetheless, they found a partial infertile results in rats. But in other aspect a point that might be worthy of notice is that there was still about 55% of the fertility testing.
Phytochemical studies indicate that the MC contains free eugenol, eugenol acetate, caryophyllene, sesquetrepene ester, phenyl propanoid, β caryophyllene, acetyle eugenol (Adewale et al., 2014; Bai et al., 2016). However, the compounds had not been scientifically studied for their effect on sexual function. The earlier study using hydro alcoholic extract (50%) of MC demonstrated the aphrodisiac activity (Tajuddin et al., 2004). Recently, the promoting effect of herbal extract on the reproduction was reported (Lee et al., 2013). But it needs further study for which ingredients have those effects and how they act within the body.
On the other hand, it is also not irrational that the ingredients of the MC extract would be converted into another substances by the metabolic process and work on the reproductive endocrine system. But it is not yet known which elements of the MC extract operate like those mentioned above. It could be greatly helpful to further examine the hormones in blood and the expression aspects of genes to uncover those action mechanisms.
The reproductive activities of golden hamsters is also controlled by gonadotropin releasing hormone (GnRH) as other mammals. And it has been well documented that the GnRH neurons in hypothalamus is indirectly affected by the melatonin, which is a major hormone secreted from the pineal gland (Choi, 2013). The synthesis and release of melatonin is proportional to the length of nighttime, thus melatonin is secreted for expanded period of time in winter, which is considered as an apparent involuting factor on reproductive activity in the golden hamsters.
For successful fertility, normal structure and accurate function of all parts of reproductive system are needed. A complex mechanism under the regulated function of the hypothalamic-pituitary-gonadal axis (HPG axis) is responsible for initiation and maintenance of spermatogenetic activity. Initially, GnRH in hypothalamus is produced and secreted to the pituitary that releases FSH and LH. FSH and LH acts on the testes. In the testes, LH stimulates the Leydig cells, located in interstitial tissue of seminiferous tubules. Then the cells produce and secrete testosterone. Simultaneously, FSH supports the function of sertoli cells, a mediator for effects of testosterone and exerts its effect on germ cells for successful spermatogenesis in seminiferous tubules.
The action mechanism of the MC extract could be speculated as follows no matter what animals were used. The MC extract is thought to act on the reproductive endocrine system by exerting its effect on GnRH neuronal cells. The MC extract swallowed by intubation into the animal body could be absorbed in the gastrointestinal tract and then spreaded all over the body through the circulatory system. Thus some compounds could act on hypothalamus, ultimately on the cells generating GnRH. Also in a similar manner, they may apply directly to the pituitary. But the evidence has not been assured.
Despite of the effect of SP on reproduction of the animals, the consequence of complete ineffectiveness by MC treatment could be related to the release of melatonin (Stetson & Watson-Whitmyre, 1984; Stetson & Watson-Whitmyre, 1986). That is, MC extract somewhat does not change the period of secretion of melatonin leading to involution, although the pattern of melatonin secretion was not examined. If that is the case, the MC extract is thought to nullify any modification of the gonadotropin releasing hormone (GnRH) neuronal cells via melatonin to influence the release of FSH and LH from the anterior pituitary, which exert their effects on testicular function.
It is known that SP reduces the release of GnRH via the melatonin. Thus the MC extract possibly impinge on the action of melatonin, resulting in functional involution of reproductive activity in this animal. Moreover it can be speculated that they affect directly the testes as emphasized by the previous report (Tumkiratiwong et al., 2014). The components of the MC extract would operate on the Leydig cells to suppress the production of testosterone, and then the reduced amount of steroid influences the spermatogenesis. If it happened to golden hamsters, they should have had small regressed testes. In light of large testes that animals treated with MC (low and high dosages) displayed, it is resonable that MC extract had no direct influences on testes. The outcome that MC extract was impotent on the reproductive activity of golden hamsters was evidenced by the offsprings who were born from the animals received the MC extract. Their pregnant ratio was similar to the LP controls, showing near 60% in generating offsprings. While SP control animals had no litters at all, implying complete degenerating effects of SP on testicular function.
In conclusion, the administration of MC extract did not alter the photoperiodic effects of LP on the reproduction of male golden hamsters. These results rule out the direct influence of MC on the testes, suspecting the antifertility action of MC presented in other animals. Thus MC extract could act on the reproductive activities via hypothalamopituitary gonad axis, including melatonin in the case of golden hamster. But it needs further study for how the natural product regulates the reproductive endocrine system within the body.

