INTRODUCTION
The epididymis of the male reproductive tract is the place where sperm produced from the testis becomes mature and acquires fertilizing capacity. The epididymis has a layer of epithelial lumen surrounded by smooth layers, and the epididymal epithelium is composed of several cell types, for example principal, halo, clear, basal, and apical cells, possessing distinctive functions (Robaire & Hermo, 1988) Based on histochemical and functional aspects, the epididymis is divided into four parts, initial segment, caput epididymis, corpus epididymis, and caudal epididymis (Robaire & Hermo, 1988). Of these epididymal regions, the caput epididymis has some features distinct from other parts of the epididymis. These include different composition of epithelial cell types (Robaire & Hermo, 1988), thickness of the epithelium (Robaire & Hermo, 1988), and different expressional levels of certain molecules, such as androgen receptor and 5α-reductase (Robaire et al., 2006). Thus, it is generally acknowledged that the caput epididymis gives an influence on sperm maturation in ways different from other epididymal segments.
In multicellular tissue, Cellular communication between adjacent cells is mostly directed by one or more types of junctional complexes, such as tight junction, adherens junction, and gap junction (Lawrence et al., 1978). However, gap junctional complex only allows the functional accordance of neighboring cells by direct exchange of small molecules, including signaling molecules, ions, and some metabolites (Valiunas et al., 2005). The gap junction is composed of six connexin (Cx) subunits, forming a channel between contiguous cells (Lawrence et al., 1978). To date, 21 Cx isoforms have been found in vertebrates, and all cells virtually possess one type of Cx isoforms at least (Goodenough & Paul, 2009). Many researches have demonstrated that certain Cx isoforms are frequently found in most of tissues and others are expressed in restricted cell types (Goodenough & Paul, 2009; Meşe et al., 2007). Other studies have also showed co-expression of more than two types of Cx isoforms and differential expression of several Cx isoforms during the development in a cell (Goodenough & Paul, 2009; Meşe et al., 2007). The existence of Cx isoforms in the male reproductive tract has been extensively examined. The expression of Cx isoforms in the epididymis has been determined by our previous researches, and the expression of a total of 9 Cx isoforms, including Cx26, Cx30.3, Cx31, Cx31.1, Cx32, Cx37, Cx40, Cx43, and Cx45, have been identified in the epididymis (Han & Lee, 2013; Lee, 2013; Seo et al., 2010). Moreover, these Cx isoforms in the epididymis have been differentially expressed according to the segment and/or age during postnatal development (Seo et al., 2010; Han & Lee, 2013; Lee, 2013). The localization of Cx43 between basal and principal cells in the rat caput epididymis has been detected by Cyr et al (1996). However, addition information for the localization of other Cx isoforms in the epididymis is limited to date.
The expression of Cx isoforms in the epididymis is regulated by various intrinsic and/or extrinsic factors. The expression of Cx43 in the rat caudal epididymis is controlled by testis-originated factor(s) because the removal of testis results in the disappearance of immunoreactivity of Cx43 (Cyr et al., 1996). In addition, the expressional stimulation of Cx43 in human epididymis by epidermal growth factor has been reported (Dubé et al., 2012). Propylthiouracil-induced hypothyroidism at the neonatal age induces a significant decrease of Cx43 expression in the entire epididymal region, except caudal epididymis, indicating a possible role of thyroid hormone on expression of Cx43 in the epididymis (St-Pierre et al., 2003). It is well-documented that the function of epididymis is largely regulated by androgen, as well as estrogen (Robaire & Hamzeh, 2011; Schulster et al., 2016). Thus, the possibility of expressional regulation of Cx isoforms in the epididymis by androgen and/or estrogen-related compounds has been suggested. Indeed, the exposure to flutamide (Flu), an anti-androgenic substance, at the late gestational age or early postnatal age causes a decrease of Cx43 expression in the cauda epididymis (Lydka et al., 2011). In addition, our previous researches have demonstrated the effects of flutamide and estradiol benzoate (EB), an estrogenic compound, treatments at the early neonatal age on expression of Cx isoforms within different epididymal segments (Lee, 2015, 2016a, 2017). Moreover, the exposure of these substances at different postnatal age results in differential expression of Cx isoforms within corpus or caudal epididymal region (Lee, 2015, 2016a, 2016b; Lee & Lee, 2015). Recently, after the exposure to Flu or EB at the early neonatal age, expressional changes of Cx isoforms in the adult caput epididymis have been evaluated (Lee, 2017). But, the influence of Flu or EB treatment at different age on expression of Cx isoforms in the caput epididymis has not been studied. Thus, the aim of the present research was to evaluate the changes of expression of Cx isoforms in the adult caput epididymis treated with Flu or EB at the weaning.
MATERIALS AND METHODS
Pregnant Sprague Dawley female rats were purchased from Samtako (OSan, Korea) and were separated in individual until the delivery and weaning of pups. The pregnant rats were randomly assigned into one of five experimental groups, including control group treated with peanut oil (n=6), a low-dose EB [EB-L, 0.015 μg of EB/kg body weight (BW), n=6] treated group, a high-dose EB (EB-H, 1.5 μg of EB/kg BW, n=6) treated group, a low-dose Flu (Flu-L, 500 μg of Flu/kg BW, n=6) treated group, and a high-dose Flu (Flu-H, 5 mg of Flu/kg BW, n=6) treated group. The animal was allowed to freely access food and drinking water during the entire experimental period.
To prepare EB or Flu solution to be injected, the powder forms of EB and Flu were obtained from Tokyo Chemical Industry Co. (Tokyo, Japan). The adequate amount of EB or Flu was completely dissolved in 100% EtOH, and the concentrated solution was then diluted in peanut oil to get working solution of EB or Flu. At the weaning, the animal was weighted and was subcutaneously administrated with a proper amount of EB or Flu working solution. The volume of injected EB or Flu solution or peanut oil did not exceed 0.05 mL. The current work was conducted in accordance with the guide for the care and use of laboratory animals of National Research Council in S. Korea.
The experimental animal at 4 months of age was euthanized by CO2 stunning, and an incision on lower abdomen was made to take out the male reproductive tract. In the cold PBS buffer, the epididymis was separated from the rest and was additionally divided into individual epididymal parts. The caput epididymis was transferred to and washed in new cold PBS buffer and was rapidly placed in liquid nitrogen. Frozen tissue was stored in –80℃ and was used for total RNA isolation.
The extraction of total RNA was began with the homogenization of tissue in the RNA extraction solution (iNtRON Biotech, Sungnam, Korea). The precipitation of total RNA was carried out with the addition of isopropanol. After the washing in 70% DEPC-EtOH, total RNA pellet was air-dried and then resuspended in DEPC- dH2O. The amount and purity of total RNA isolated were calculated with NanoDrop Lite spectrophotomer (Thermo Scientific, Wilmington, DE), and the qualitative condition of total RNA was checked by 1.2% agarose gel electrophoresis.
A mixture of 1 μg of total RNA, oligo-dT primer, ImProm- IITM reverse transcription system (Promega, Madison, WI), and dH2O in a total volume of 20 μL was prepared to construct the first-stranded complementary DNA (cDNA). The mixture was placed in a sequential step of 25℃ for 5 min, 42℃ for 90 min, and 70℃ for 15 min to carry out the reverse transcription reaction. The generated cDNA was utilized as a template DNA for quantitative real- time PCR analysis.
A mixture of 1 μL of cDNA, 10 pmol of each primer, 10 μL PCR master mixture (Finnzymes, Espoo, Finland), and DNase-free dH2O in a final volume of 20 μL was made for quantitative real-time PCR analysis of each Cx isoform. Table 1 shows detailed information of oligonucleotide primers and condition used for real-time PCR analysis. The PCR was undertaken in a thermocycler (Bio-Rad Laboratories, Hercules, CA) with a series of steps as followings; a pre-denaturation step at 95℃ for 30 sec, 35 cycles of denaturation at 95℃ for 30 sec, annealing at Tm for 30 sec, and extension at 72℃ for 30 sec, and a final extension step at 72℃ for 5 min. Cyclophilin A (Ppia) was used for an internal control of quantitative real-time PCR.
The RT reactions and PCRs were independently quadruplicated to get a mean and a standard error of an experimental group. The experimental results were reported in the relative ratios of expression between Ppia and Cx isoforms. Statistical significance of Cx expressional level among control and two experimental groups of EB or Flu treatment was determined by one-way ANOVA, followed by Duncan’s test. If P < 0.05, it was considered that there was a statistical difference between two groups.
RESULTS
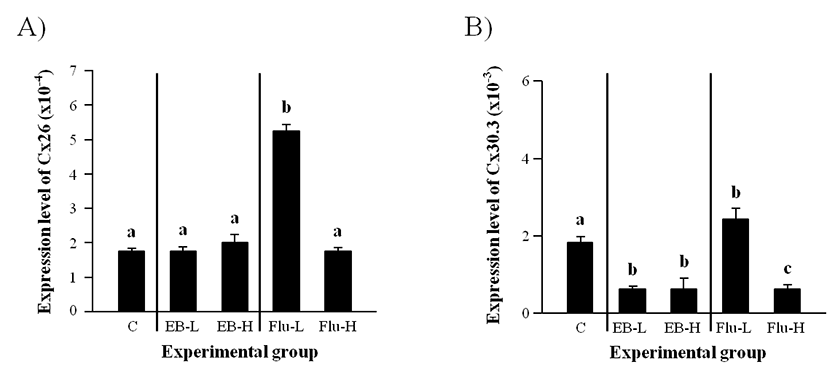
As shown in Fig. 1A, there was no significant change of Cx26 transcript level with EB treatment at both low and high doses. However, a significant increase of Cx26 expression was detected with a low-dose treatment of Flu (Fig. 1A). The treatment of Flu at a high-dose didn’t give a significant influence on the level of Cx26 transcript.
Expression of Cx30.3 in the adult caput epididymis was significantly decreased by a low-dose EB treatment at the weaning (Fig. 1B). Even though a high-dose EB treatment also caused a decrease of Cx30.3 transcript level, there was no difference on Cx30.3 transcript level between a low-dose and a high-dose EB treatment (Fig. 1B). A significant induction of Cx30.3 expression was observed with a low-dose Flu treatment, but a high-dose Flu treatment at the weaning resulted in a decrease of Cx30.3 mRNA level in the adult caput epididymis (Fig. 1B).
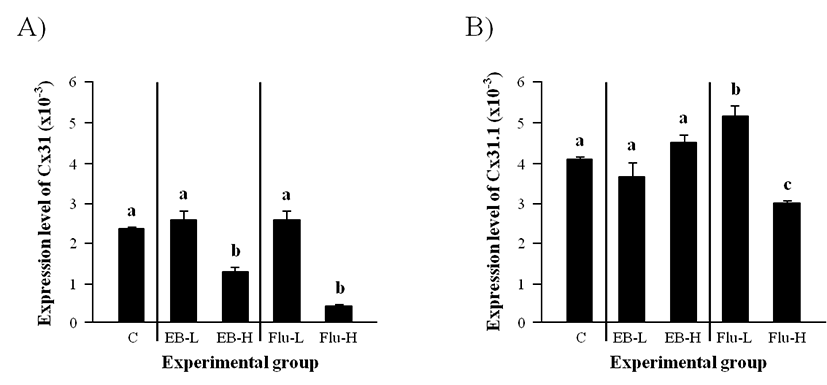
The level of Cx31 transcript in the adult caput epididymis was not changed by the administration of a low-dose EB at the weaning (Fig. 2A). But, a significant reduction of Cx31 transcript level was observed with a high-dose EB treatment (Fig. 2A). Similarly, the treatment of a low-dose Flu at the weaning didn’t affect the expression of Cx31 in the adult caput epididymis, while the expression of Cx31 was greatly decreased by the treatment of a high-dose Flu (Fig. 2A).
Neither a low-dose EB treatment nor a high-dose EB treatment gave any significant effect on the expressional change of Cx31.1 (Fig. 2B). The administration of a low-dose Flu at the weaning caused a clear increase of Cx31.1 transcript level in the adult caput epididymis (Fig. 2B). But, a significant decrease of Cx31.1 mRNA abundance in the adult caput epididymis was detected in the high-dose Flu treated-group (Fig. 2B).
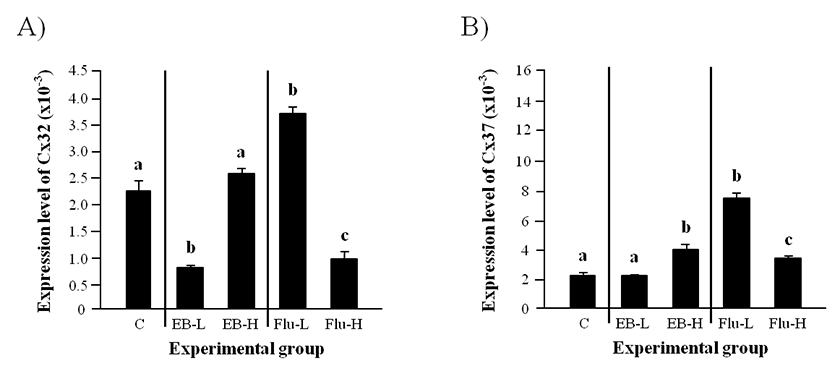
A significantly lower level of Cx32 in a low-dose EB treated-group at the weaning than that in the control was detected in the adult caput epididymis, even though there was no significant change of Cx32 transcript level in a high-dose EB treated-group (Fig. 3A). The level of Cx32 transcript was significantly increased by a low-dose Flu treatment, but the treatment of a high-dose Flu led to a significant decrease of Cx32 transcript level (Fig. 3A).
Expression of Cx37 in the adult caput epididymis was not changed by a low-dose EB treatment at the weaning (Fig. 3B). However, the treatment of a high-dose EB at the weaning resulted in a significant induction of Cx37 expression (Fig. 3B). The treatment of Flu at a low-dose caused a significant increase of Cx37 transcript level (Fig. 3B). The expressional level of Cx37 in a high-dose Flu treated-group was significantly higher than that in control but lower than that in a low-dose Flu treated-group (Fig. 3B).
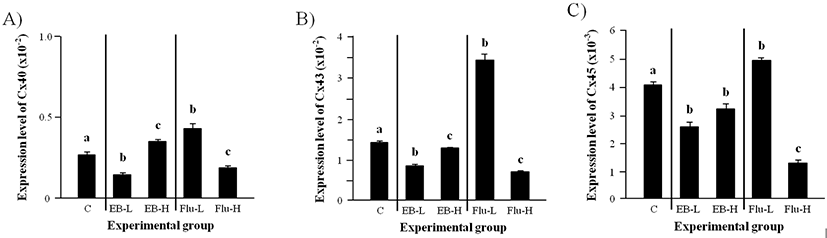
The transcript level of Cx40 in the adult caput epididymis was significantly decreased by a low-dose EB treatment at the weaning (Fig. 4A). However, the exposure to a high-dose EB at the weaning resulted in a significant increase of Cx40 mRNA level (Fig. 4A). An increase of Cx40 transcript level in the adult caput epididymis was detected by a low-dose Flu treatment at the weaning, while the treatment of a high-dose Flu caused a significant decrease of Cx40 mRNA amount (Fig. 4A).
The expression of Cx43 in the adult caput epididymis was significantly decreased by both low- and high-dose EB treatments at the weaning (Fig. 4B). But, the level of Cx43 transcript was significantly lower in a low-dose EB treated-group than a high-dose EB treated-group (Fig. 4B). A great increase of Cx43 mRNA level in the adult caput epididymis was observed with a low-dose Flu treatment at the weaning (Fig. 4B). In the other hand, a high-dose Flu treatment caused a decrease of Cx43 transcript level (Fig. 4B).
The treatments of both low- and high-dose EB at the weaning resulted in significant reduction of Cx45 trans- cript level in the adult caput epididymis (Fig. 4C). An increase of Cx45 expression in the adult caput epididymis was found in a low-dose Flu treated-group, but the treatment of a high-dose Flu at the weaning resulted in a significant decrease of Cx45 transcript level in the adult caput epididymis (Fig. 4C).
DISCUSSION
The expression of Cx isoforms in the adult rat caput epididymis after the exposure to Flu or EB at the weaning has been examined in the present study. Even though expressional changes of Cx isoforms by the EB treatments at two doses are somewhat not uniformed, in general, a low-dose Flu treatment causes expressional increases of most Cx isoforms and a high-dose Flu treatment results in expressional decreases of most Cx isoforms in the caput epididymis.
Aberrant expression of Cx isoforms in the adult corpus and caudal epididymis after Flu or EB treatment at the weaning has been reported from our previous researches (Lee & Lee, 2015; Lee 2016b). Each Cx isoform has shown different responses to Flu or EB treatment at the weaning age according to the epididymal region and/or dose of treatment. For example, expression of Cx26 in the adult corpus epididymis is significantly increased by a high-dose EB treatment but significantly decreased by a high-dose Flu treatment (Lee & Lee, 2015). With the same treatment, in the adult caudal epididymis, expression of Cx26 is not changed by EB treatment but significantly increased by Flu treatment at two doses (Lee, 2016b). Different expressional response of Cx43 has been observed in corpus and caudal epididymis after the same Flu or EB treatment. That is, expression of Cx43 in the adult corpus epididymis is significantly increased by a low-dose EB treatment but decreased by a high-dose EB and two-doses Flu treatments (Lee & Lee, 2015). Interestingly, expression of Cx43 in the adult caudal epididymis is significantly increased by EB or Flu treatment at the weaning age (Lee, 2016b). In the present study, the EB treatment has not affected the expression of Cx26, but a low-dose Flu treatment results in an increased of Cx26 expression. Expression of Cx43 is significantly decreased by EB treatment, but a low-dose Flu treatment results in a significantly increase of Cx43 expression, while a significantly reduction of Cx43 expression is detected by a high-Flu treatment. Together, these observations suggest that expression of each Cx isoforms in the epididymis is differentially regulated during postnatal development in a segment-specific manner.
Abnormal expression of Cx isoforms in the adult epididymis by EB or Flu treatment are likely influenced by the time of exposure during the early postnatal development. Our earlier researches have showed differential expressional patterns of each Cx isoforms within the adult corpus or caudal epididymis after the EB or Flu treatment at 1 week or 3 weeks of postnatal age (Lee & Lee 2015; Lee 2015, 2016a, 2016b). The comparison of the findings of current research and a previous study (Lee 2017) in the caput epididymis also supports the view that expression of Cx isoforms in the adult epididymis could be differentially appeared according to the time of contract to exogenous substance. Indeed, Ma et al (2017) have demonstrated differential gene expression in the adult rat spleen after the exposure to fluoride at different postnatal age. In addition, the exposure to N-methyl-N-nitrosourea at different age during pre- and post-pubertal period results in differential expression of COX2 in granule cells, affecting pattern of normal neurogenesis in rat hippocampus (Watanabe et al., 2017). Therefore, it is also acceptable that EB or Flu treatment at different postnatal age could result in differential expression of Cx isoforms.
A couple of explanations about what causes such differential expression of Cx isoforms in the adult epididymis after EB or Flu treatment at different postnatal age are suggested. One explanation is related with differentiation of epididymal epithelial cells during postnatal period. The undifferentiated epithelial cells in the epididymis at the birth undergo a large histological change during the early infancy, and the first differentiated cell, halo cell, appears at the infancy (Arrotéia et al., 2012). After the appearance of halo cell, until the puberty, the undifferentiated epididymal epithelial cells become first differentiated into columnar or non-columnar cells (Arrotéia et al., 2012). Then the columnar cells develop into principal and basal cells and noncolumnar cells are differentiated into apical, narrow, and clear cells over postnatal time (Arrotéia et al., 2012). Thus, if EB or Flu treatment is carried out at different postnatal age, it is considered that the treatment could influence on the differentiation of epididymal epithelial cell and thereby result in disruption of proper development of epithelial cells in the adult epididymis. This phenomenon could end up with aberrant expression of cellular molecules, including Cx isoforms, and consequently functional abnormality of the epididymis. The other possibility to describe differential effects of EB or Flu treatment at different postnatal age on expression of Cx isoforms in the epididymis is due to differential expression of androgen receptor (AR) and estrogen receptor (ER) in the epididymis during postnatal period. The Flu is a pure anti-androgen acting with AR to regulate expression of androgen-responsive genes (Tan et al., 2015). The expression of AR in the epididymis is elevated during postnatal period, especially with a surgical increase of AR expression for the early postpartum period (You & Sar, 1998). The EB is an estrogen-agonistic compound acting through ER to regulate gene expression (Serova et al., 2010). The expression of ERα in the epididymis during postnatal development is under the regional- and age-specific patterns, and the exposure to diethylstilbestrol leads into disrupted expression of ERα in the epididymis (Atanassova et al., 2001). These earlier observations would support that the treatment of EB and/or Flu at different postnatal age could manipulate expression of AR or ER in the adult epididymis and thus cause abnormal expression of androgen- and/or estrogen-responsive genes, such as likely Cx isoforms. Detailed molecular examination on expressional regulation of Cx isoforms in the epididymis by androgen and estrogen should be conducted in future.
In conclusion, the present research exhibits that expression of Cx isoforms in the adult caput epididymis is modulated by the treatment of EB or Flu at the weaning age. In addition, the data from the current study also show that the EB or Flu treatment at different postnatal age results in differential expression of a given Cx isoform in the adult caput epididymis. However, it still remains to be answered if the abnormal expression of Cx isoforms in the epididymis by EB or Flu treatment is related with a disruption of epididymal function.

