INTRODUCTION
The epididymal fat is a gonadal white adipose tissue and is found between the testis and head of epididymis (Niemalä et al., 2008). Even though the epididymal fat-like structure in the mouse is observed from the birth, fully mature adipocyte of the epididymal fat is detectable from 14 days of postnatal age (Han et al., 2011). The epididymal fat is consisted of not only preadipocytes and mature adipocytes but also various types of cells, including immune and endothelial cells (Cleary Margot et al., 1977). Like other types of white adipose tissue, the epididymal fat continuously grows and expends in mass and size during postnatal period, accompanying a changes of cellular compositions (Cleary Margot et al., 1977; Björntorp et al., 1979). The epididymal fat is divided into two regions, proximal and distal regions, depending on histochemical characteristics and the proximity to the testis (Tirard et al., 2007). But Altintas et al. (2011) have divided the epididymal fat into three parts relative to the location of spermatic blood vessels and have reported expression of several adipocytokines and the existence of different cellular compositions among these epididymal parts. These observations suggest the existence of regional differences within the epididymal fat, in the respect of behaviors and/or presumably functions.
Expression of adipogenic molecules in different epididymal fat parts during postnatal period has been examined (Lee, 2019; Lee & Kim, 2019). These researches have shown that expression patterns of adipogenic molecules differ between the epididymal fat regions according to postnatal age, even though the tendency of increased expression levels of adipogenic molecules has been observed with ages, in accordance with a steady growth of the epididymal fat during postnatal period (Lee, 2019; Lee & Kim, 2019). In addition, expression of several adipocytokines, including leptin, adiponectin, and resistin, in the epididymal fat at different postnatal ages has been evaluated (Lee & Kim, 2018; Lee, 2019; Lee & Kim, 2019). Similar with those of adipogenic molecules, expression of adipocytokines in the epididymal fat seems to be increased with postnatal age, in spite of distinct expression pattern of each adipocytokine during postnatal period (Lee & Kim, 2018; Lee, 2019; Lee & Kim, 2019). Thus, it is likely that differential expression of adipocytokines in the epididymal fat during postnatal period is accompanied with growth and development of the epididymal fat.
It is well recognized that adipose tissue is a highly active endocrine organ, which expresses and secretes various kinds of adipocytokines with endocrine functions (Kershaw & Flier, 2004). Many researches have determined the existence of receptors for adipocytokines in the testis, including leptin receptor (Tena-Sempere & Barreiro, 2002), adiponectin receptor (Ocón-Grove et al., 2008), and chemerin receptor (Li et al., 2014). In addition, the effects of adipocytokines on the testicular functions have been examined from many previous researches. Leptin induces decreases of steroidogenic acute regulatory protein (Star) and cytochrome P450 side chain cleavage (Cyp11a1) expression in the testis, thus resulting in reduction of testosterone production (Tena-Sempere & Barreiro, 2002). Chemerin also suppresses the production of testosterone by causing a decrease of 3 beta- and steroid delta-isomerase (Hsd3b) expression in the testis (Li et al., 2014). Moreover, a stimulatory effect of adiponectin on testosterone production in the testis by increasing expression of Star and Cyp11a1 is found (Rak et al., 2017). These data clearly indicate that adipocytokines regulate testicular functions for testosterone production and spermatogenesis by controlling expression of steroidogenic enzymes. However, most of these effects of adipocytokines on the testis have been acquired from exogenous exposure to adipocytokines, manipulation of adipocytokine and its receptor genes, and/or induced clinical conditions, such as obesity. It is fairly difficult to find previous researches dealing with an innate influence of epididymal fat on the testicular functions. Chu et al. (2010) have demonstrated that surgical removal of the epididymal fat (EPFX) in the young adult hamster completely abolishes the spermatogenesis but not affects serum testosterone concentration. However, this research has not examined whether the EPFX gives an influence on expression of steroidogenic enzymes in the testis.
Thus the present research was aimed to find out the effect of partial EPFX on expression of testicular steroidogenic enzymes of mouse during postnatal period. Using a quantitative real-time polymerase chain reaction (PCR) analysis, the changes of expression patterns of steroidogenic enzymes in the testis by partial EPFX were evaluated and compared at different postnatal ages.
MATERIALS AND METHODS
A total of 29 male C57BL/6 male mice at 7 weeks of postnatal age were obtained from Samtako (Osan, Korea). Each mouse was separately housed from others during a whole experimental period. The animals were permitted to access to food and water ad libitum and kept in controlled environments. Once reaching at 2 months of postnatal age, the animals were divided into four experimental age groups, a young adult group (2 months), two mature adults (5 and 8 months), and an elderly adult (12 months). Each experimental group was further divided into two sub-experimental groups, sham and epididymal fat lipectomy (EPFX) groups, including sham (n=4) and EPFX (n=4) at 2 months, sham (n=3) and EPFX (n=3) at 5 months, sham (n=3) and EPFX (n=4) at 8 months, and sham (n=4) and EPFX (n=4) at 12 months of postnatal age.
The surgical EPFX was performed to the animals at proper postnatal ages. The mouse was anesthetized with ether, and a small incision on each side of scrotum was made with a pair of fine scissors and forceps. The reproductive tract, including testis, epididymis, and epididymal fat, was exposed to the outside through the incision. The careful EPEX was performed to minimalize interruption of the blood supply to the testis, and most of epididymal fat taken by EPEX was the proximal epididymal fat part. The equal surgical practice was carried out to another side of the male reproductive tracts. The scrotum incision was closed with instant glue (Aron Alpha Instant glue, Toagosei, Tokyo, Japan), and the careful observation was paid for assured recovery from the anesthetization and surgical actions. When the full recovery was confirmed, the mouse was placed back to its own cage and kept eye on any abnormal behaviors and casualty. Same surgical procedure was applied to the mouse in sham group, except EPFX.
The testis from each experimental mouse was collected after two weeks of the EPFX. The animal was euthanized by quick cervical dislocation, and the reproductive tract was isolated through an abdominal incision. In cold phosphate-buffered saline solution, the testis was rapidly separated from other parts of reproductive tract and quickly placed in liquid nitrogen. The testis was kept in −80°C freezer for further use.
Isolation of total RNA from the frozen testis was carried out with Trizol reagent (Molecular Research Center, Cincinnati, OH, USA). Chloroform and isopropanol were sequentially applied to homogenized testis in Trizol solution to isolate and precipitate total RNA. Air-dried total RNA was re-suspended in DEPC-dH2O, and quantitative measurement of isolated total RNA was performed with NanoDrop Lite spectrophotometer (Thermo Scientific, Massachusetts, MA, USA). The quality of total RNA was evaluated by 1.2% agarose gel electrophoresis.
Generation of complementary DNA (cDNA) strand from isolated total RNA was carried out with 1 μg of total RNA by using iScripTM Reverse transcription Supermix for reverse transcription (RT)-qPCR (Bio-Rad Laboratories, Hercules, CA, USA). The final volume of RT reaction was adjusted with nuclease-free dH2O to 20 μL, and the RT reaction was performed at 25°C for 5 min, 46°C for 20 min, and 95°C for 1 min.The cDNA product was directly used for a quantitative real-time PCR.
One microliter of cDNA generated was mixed with 7 μL of iQTM SYBR® Green Supermix (Bio-Rad Laboratories), 10 pmol of each oligonucleotide primer, and nuclease-free dH2O to adjust to a total volume of 25 μL. The examined steroidogenic enzymes include steroidogenic acute regulatory protein (Star), cytochrome P450 side chain cleavage (Cyp11a1), cytochrome P450, family 17, subfamily A, polypeptide 1 (Cyp17a1), hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 (Hsd3a1), and hydroxysteroid 17-beta dehydrogenase 3 (Hsd17b3), and the primer information utilized for real-time PCR is shown in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used for an internal control molecule for PCR analysis. The PCR condition was as follows, a pre-denaturation step at 95°C for 5 min, cycles of a denaturation step at 95°C for 30 sec, an annealing step at Tm for 30 sec, and an extension step at 72°C for 30 sec in PTC-200 Chromo 4 real-time system (Bio-Rad Laboratories). An additional extension step at 72°C for 10 min was added at the end of each PCR. The sizes of PCR products were confirmed by 1.2% agarose gel electrophoresis.
PCR, polymerase chain reaction; Star, Steroidogenic acute regulatory protein; Cyp11a1, cytochrome P450 side chain cleavage; Cyp17a1, cytochrome P450, family 17, subfamily A, polypeptide 1; Hsd3a1, hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1; Hsd17b3, hydroxysteroid 17-beta dehydrogenase 3; and Gapdh: glyceraldehyde-3-phosphate dehydrogenase.
Independently triplicated RT reactions and real-time PCRs were performed to obtain a mean for each experimental animal, and the transcript levels of steroidogenic enzymes were adjusted to that of Gapdh to acquire means and standard errors of an experimental group. The data were expressed in the relative ratios of transcript levels of steroidogenic enzymes against Gapdh. The Student t-test was employed to determine statistical differences at transcript levels of steroidogenic enzymes between sham and EPFX groups at each postnatal age. If p<0.05, it was considered to be statistically significant between two groups.
RESULTS
The transcript level of Star in the testis of the EPFX mouse at 2 months of postnatal age was significantly higher than that of sham mouse (Fig. 1A). However, a significant decrease of Star transcript level was detected in the testis of the EPFX mouse at 5 months of postnatal age, compared with that in sham mouse (Fig. 1A). There was no significant change of Star mRNA abundance in the testis after the EPFX at 8 months of postnatal age (Fig. 1A). Interestingly, the EPFX at 12 months of postnatal age resulted in a significant increase of the abundance of testicular Star mRNA, compared to the level of sham group (Fig. 1A).
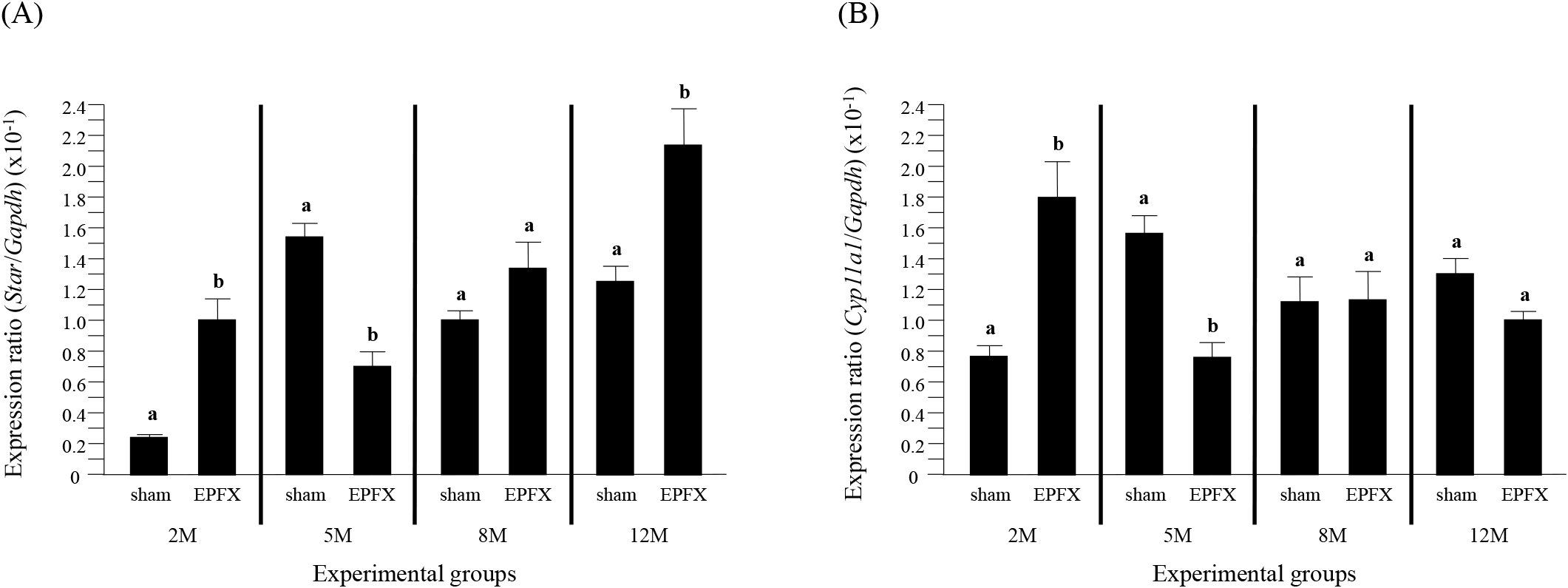
The expression of Cyp17a1 in the mouse testis after the EPFX at 2 months of postnatal age was significantly increased, compared to that in sham group (Fig. 2A). However, a transient decrease of Cyp17a1 transcript level was detected in the testis of EPFX group at 5 months of postnatal age (Fig. 2A). A significant decrease of Cyp17al mRNA abundance in the testis of EPFX group was also found at 8 months of postnatal age, while there was no significant change of Cyp17a1 expression level in the testis of EPFX group at 12 months of postnatal age (Fig. 2A).
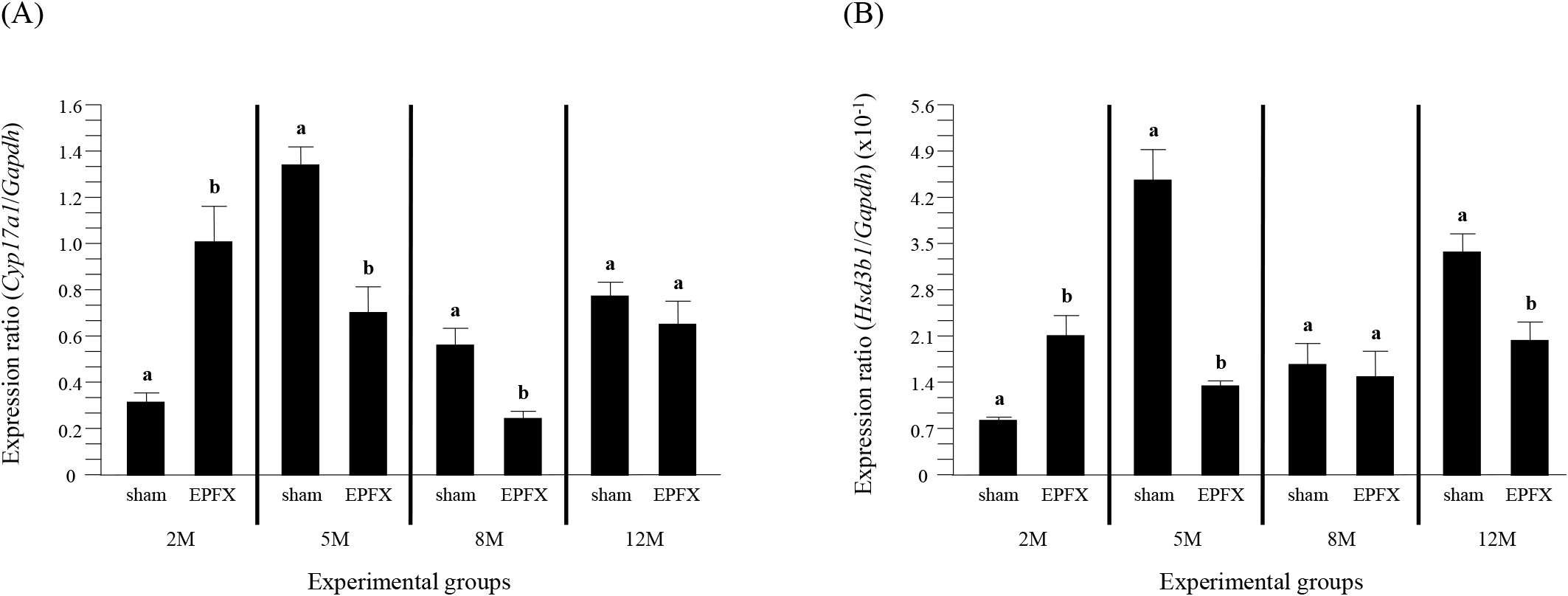
The EPFX at 2 months of postnatal age resulted in a significant increase of Hsd3b1 transcript level in the testis, compared to that in the testis of sham group (Fig. 2B). The mRNA abundance of the testicular Hsd3b1 in the testis of EPFX mouse at 5 months of postnatal age was significantly lower than that of sham group (Fig. 2B). At 8 months of postnatal age, the EPFX did not give a significant influence on the level of the testicular Hsd3b1 transcript (Fig. 2B). A significant decrease of Hsd3b1 transcript level in the testis of EPFX mouse, compared to that of sham group was also found at 12 months of postnatal age (Fig. 2B).
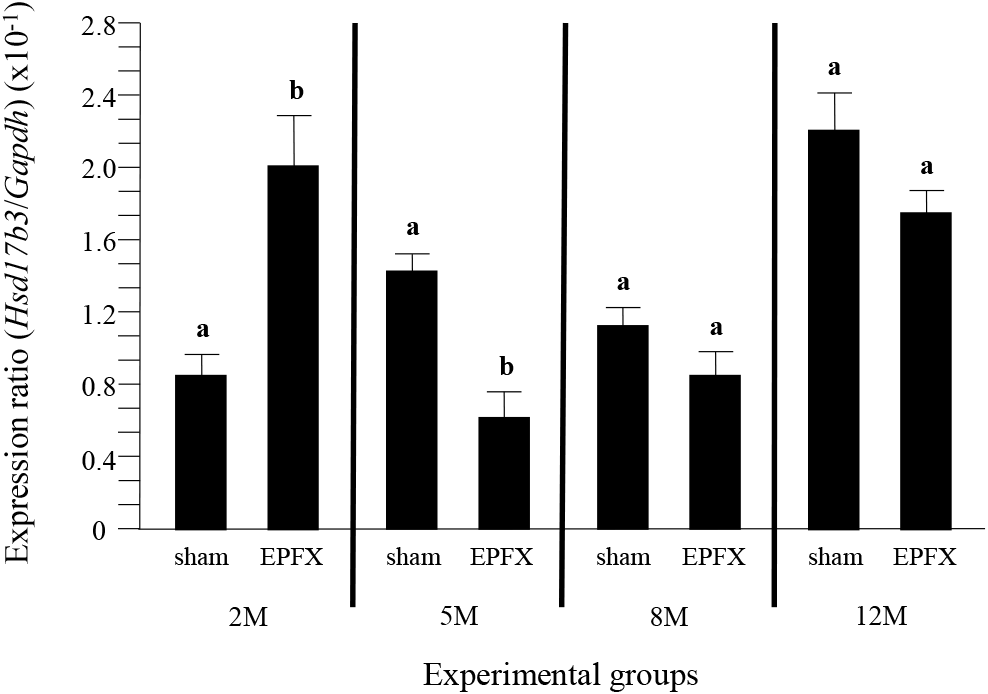
The expression level of Hsd17b3 in the testis of EPFX mouse at 2 months of postnatal age was significantly increased, compared to that of sham mouse, while the level of Hsd17b3 transcript in the testis of EPFX mouse was significantly decreased at 5 months of postnatal age (Fig. 3). At 8 months of postnatal age, the EPFX did not cause the change of Hsd17b3 expression level in the mouse testis, and a similar observation was detected at 12 months of postnatal age (Fig. 3)
DISCUSSION
Partial removal of the epididymal fat by surgical procedure at different postnatal age resulted in differential expression of steroidogenic enzymes in the testis, and the findings are as follows: 1) expression of all steroidogenic enzymes evaluated is significantly increased after EPFX at 2 months of postnatal age; 2) the EPFX caused significant decreases of transcript levels of testicular steroidogenic enzymes by EPFX at 5 months of postnatal age; and 3) the effect of EPFX at 8 and 12 months of postnatal ages on expression of steroidogenic enzymes in the testis is not likely prominent, even though there are some steroidogenic enzymes displaying significant changes on their expression levels.
Expression of adipocytokines in the epididymal fat has been examined by our and other researches. Altintas et al. (2011) have shown the existence of various adipocytokine transcripts in the mouse epididymal fat, including leptin, adiponectin, tumor necrosis factor (TNF)-α, and interleukin (IL)-6 and 10. Expression of resistin in the epididymal fat is also reported from a previous research (Ye et al., 2013). Our earlier studies have also shown expression patterns of leptin, adiponectin, and resistin transcripts in the epididymal during postnatal period (Lee, 2019; Lee & Kim, 2019). Notably our previous researches have displayed that expression patterns of these adipocytokines in distal and proximal epididymal fat parts are somewhat different from each other, especially at 5 months and 12 months of postnatal ages (Lee, 2019; Lee & Kim, 2019). In addition, others have demonstrated secretion of adipocytokines from the epididymal fat. Secretion of leptin from the epididymal fat tissue culture has been reported, and the amount of leptin secretion is higher at young adult than at immature animal (Nazian, 2013). Resistin secretion from the epididymal fat has been confirmed by ex vivo epididymal fat transplant culture (Ye et al., 2013). Together, these findings suggest that the epididymal fat could act as an endocrine tissue which releases various types of cytokines that might play a regulatory role in development and function of the male reproductive tract, including testis and epididymis. However, a direct effect of the epididymal fat on the male reproductive tract has been rarely examined, and the present research would offer the data indicating the possible functional role of the epididymal fat-derived adipocytokines on expressional regulation of steroidogenic enzymes in the testis.
The regulatory effects of adipocytokines on the testicular steroidogenesis by modulating expression of steroidogenic enzymes have been investigated by several researchers. Inhibitory effects of leptin and chemerin on expression of Star, Cyp11a1, and Hsd3b, accompanied with reduction of testosterone, in the testis have been examined (Tena-Sempere & Barreiro, 2002; Li et al., 2014), which adiponectin stimulates expression of testicular Star and Cyp11a1 to induce the production of testosterone (Rak et al., 2017). The stimulatory effect of resistin on testosterone production has been determined by Nogueiras et al. (2004). However, these researches have applied with artificial conditions, such as exogenous exposure to adipocytokines and/or in vitro culture methods, and thus the outcomes from these researches would not be directly relevant to the effect of epididymal fat-derived adipocytokines on the testis. The influence of innate epididymal fat on the testicular functions has been determined by surgical lipectomy of epididymal fat (Chu et al., 2010). The EPFX causes the arrest of normal spermatogenesis but does not impact on testosterone level in serum (Chu et al., 2010). But, Chu et al. (2010) have not provide any information about expression of testicular steroidogenic enzymes by the EPFX. Our present study has shown differential responses on expression of steroidogenic enzymes in the testis to EPFX at different postnatal ages. Especially, the expressional responsiveness of testicular steroidogenic enzymes to EPFX is more notable at 2 and 5 months than at 8 and 12 months of postnatal ages. These observations imply that expression of steroidogenic enzymes in the testis at younger age would be more susceptible to epididymal fat-derived adipocytokines than at old age. Moreover, the opposite expressional changes of steroidogenic enzymes at 2 and 5 months of postnatal ages suggest the existence of distinct regulatory mechanisms for expression of these testicular enzymes at two different postnatal ages.
Even though the current research has provided an evidence indicating the direct influence of the epididymal fat to expression of testicular steroidogenic enzymes for the first time to our knowledge, there are several issues to be considered for estimate of the physiological effect of epididymal fat on the testicular functions. Surgical removal of entire epididymal fat is almost impossible due to the distribution of innervation and blood systems present within the epididymal fat. The removed epididymal fat by EPFX in the present research is mostly the proximal, not distal, epididymal fat part. Thus, it is reasonable to consider that the findings from current study would be the effect of proximal epididymal fat part. The treatment of leydig cells with conditioned media collected from in vitro culture of distal or proximal epididymal fat part would provide an answer for regional effects of epididymal fat to the testis. In addition, a distinct responsiveness on expression of testicular steroidogenic enzymes to EPFX at different postnatal age should be further considered. The variable expression patterns of adipocytokine’ receptors in the testis during postnatal period could be a possible suggestion. Moreover, a physiological effect of individual adipocytokine on the testis should be distinguished from others. Expressional inhibition of specific adipocytokine gene in the epididymal fat would give informative data for a defined role of the adipocytokine on regulation of the testicular functions, including steroidogenesis.
In summary, the current research has presented that the EPFX induces distinct expression patterns of testicular steroidogenic enzymes at different postnatal ages. Additional researches are suggested to clarify detailed physiological impact of the epididymal fat on the testicular functions during postnatal period.







