INTRODUCTION
Exposure to environmental chemicals, including endocrine-disrupting chemicals (EDCs), during the gestational period can have profound adverse effects on several organs in offspring (Bernal & Jirtle, 2010; Bonde et al., 2016; Braun, 2017; Dolores Gómez-Roig et al., 2021). Some of these effects, such as disease susceptibility, may be inherited by subsequent generations, suggesting the involvement of epigenetic modifications such as DNA methylation and histone acetylation in a single sperm genome (Kumar & Thakur, 2017; Rorabaugh, 2021; Amrom & Schwartz, 2023). The heightened vulnerability of the gestational and perinatal periods to these chemicals is attributed to global re-methylation that occurs after implementation during fetal development (Reik et al., 2001; Morgan et al., 2005; Messerschmidt et al., 2014). Consequently, environmental exposures, such as EDCs, can alter DNA methylation at all stages of fetal development (Chang et al., 2006; Skinner, 2014; Walker, 2016). Gestational exposure may result in soma-wide changes, whereas exposure during adulthood may trigger more tissue-specific changes. Hence, for transgenerational epigenetic studies, the period encompassing gonadal sex determination and gonadal development (between embryonic days 8 and 15 in rats, corresponding to the 2nd trimester in humans) is experimentally selected (Anway et al., 2005).
Bisphenol A (BPA), also known as 2,2-bis-(4-hydroxyphenyl)propane, is a plasticizer produced in large quantities and primarily used in the production of polycarbonate plastics. BPA is known to leach from various sources, including epoxy resins lining metal food cans, polycarbonate baby bottles, beverage containers, dental sealants, composites, polyvinylchloride plastics, and recycled thermal paper. It can infiltrate the human body through food and drinks, and its metabolites can cross both the placental and the blood-brain barriers (Schönfelder et al., 2002; Sun et al., 2002). BPA has been detected in various human body fluids, including serum (Takeuchi & Tsutsumi, 2002), urine (Calafat et al., 2007), breast milk (Ye et al., 2006), amniotic fluid (Yamada et al., 2002), ovarian follicular fluid (Ikezuki et al., 2002), and umbilical cord blood (Kuroda et al., 2003). The liver facilitates the biotransformation of BPA via glucuronidation (Yokota et al., 1999), with subsequent elimination through bile and partially via urine. BPA exposure in humans appears to be associated with the occurrence of various reproductive diseases, such as male sexual dysfunction (Li et al., 2010), female infertility (Ziv-Gal & Flaws, 2016), polycystic ovarian syndrome (Rutkowska & Rachoń, 2014), recurrent miscarriage (Sugiura-Ogasawara et al., 2005), and premature delivery (Adu-Gyamfi et al., 2022).
In this study, we aimed to investigate the effect of gestational exposure to BPA on epigenetic, biochemical, and histological modifications in the uterine tissues of F1 adult offspring rats. We initially examined global DNA methylation in DNA samples extracted from the uterus to evaluate epigenetic changes. Subsequently, the expression and localization of the progesterone receptor (PR), a key protein in the uterus, were determined using western blot and immunohistochemistry, respectively.
MATERIALS AND METHODS
BPA, hematoxylin, neutral buffered formalin (NBF), ethidium bromide were purchased from Sigma Chemical (St. Louis, MO, USA). Rsa I, MspI (HpaII) restriction enzymes were purchased from Thermo Scientific (Thermo Fisher Scientific Korea, Seoul, Korea). Complete Protease Inhibitor Cocktail Tablet was from Roche Applied Science (Mannheim, Germany). 17β-estradiol ELISA kits were purchased from IBL (Hamburg, Germany). The western enhanced chemiluminescence detection reagent was purchased from Bio-Rad (Hercules, CA, USA). Anti-progesterone receptor (H-190 and C-19), actin antibodies were obtained from Santa Cruz Biotech (Santa Cruz, CA, USA). Anti-rabbit and mouse IgG-conjugated with horseradish peroxidase were purchased from Cell Signaling Technology (Beverly, MA, USA). VECTASTAIN® Elite ABC Kit, VECTASHIELD® Mounting Medium were from Vector Laboratories (Burlingame, CA, USA).
Adult female Sprague-Dawley rats (12 weeks of age) were obtained from SamTako Bio-Korea (Osan, Korea) and bred to male rats of the same strain in the breeding facility at the Dong-A University. Vaginal plug detection was considered day 0 of pregnancy. Pregnant rats were individually housed in a climate-controlled (21±2°C) animal room at a constant 12-h light and 12-h dark cycle, with unlimited access to rat chow. All procedures were performed in accordance with protocols approved by the Dong-A University Animal Care and Use Committee. Thirty timed pregnant rats (ten per treatment group) were given intraperitoneal injections of BPA (0.01, 1, or 10 mg/kg/day) from gestational day 8–15 (GD 8-GD 15) of gestation. Gestating control mothers received vehicle alone (corn oil). Female adult rats from control and treated groups were sacrificed by carbon dioxide asphyxiation at 6 months of age (at estrus stage of the estrous cycle) and blood samples were collected using the techniques of cardiac puncture. The right uteri were removed and fixed in NBF for histological examination. The left uteri were placed in cold phosphate buffer solution (PBS) for further biochemical analysis.
To demonstrate the methylation status of genomic DNA in uterine tissues, thin slices of uterine tissues were cut with a scalpel, stored at −80°C. DNA was isolated from uterine tissues using a DNeasy Blood and Tissue kit (QIAGEN, Aarhus, Denmark). Then, isolated genomic DNA (1 μg) was incubated and digested for 15 h at 37°C with Rsa I with either methylation-sensitive (Hpa II) or insensitive (Msp I) restriction endonucleases. Heat inactivation was performed at 65°C for all enzymes, and DNA was separated electrophoretically and visualized by ethidium bromide staining.
Uterine tissues were homogenated and lysed in lysis buffer [300 mM NaCl, 0.5% Triton X-100, 50 mM Tris-HCl (pH 7.4), 25 mM NaF, 1 mM Na3VO4, 10 mM Na4P2O7, and protease inhibitor] for 30 min on ice. The lysates were centrifuged at 13,000×g at 4°C for 20 min, the supernatants were collected and protein concentration was measured using the BCA protein assay kit. 25 micrograms of protein extract with sodium dodecyl sulfate (SDS)-loading buffer [2% SDS, 100 mM DTT, 10% glycerol, 0.02% bromophenol blue, and 50 mM Tris-HCl, pH 6.8] was electrophoretically separated on a SDS-PAGE gel, and transferred onto a nitrocellulose membrane. The membranes were blocked with 5% non-fat dry milk dissolved in Tris-buffered saline buffer containing 0.05% Tween-20 at RT for 1 h. The blots were incubated with primary antibodies, followed by incubation with appropriate HRP-conjugated secondary antibodies. The signals were detected with ECL detection reagent in the LAS-4000 (Fuji, Tokyo, Japan). Actin was used as internal control for total cellular proteins.
For immunohistochemical staining of uterus, tissue sections were deparaffinized and hydrated, treated in 3% hydrogen peroxide for 5 min, and rinsed with PBS for 15 min. Sections were incubated with the primary and secondary antibodies and labeled using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions. The nuclei were counterstained with hematoxylin. For negative controls, rabbit IgG (1 mg/mL) was used instead of the primary antibodies. The results were observed using a ScanScope digital slide scanning system (Aperio Technologies, Vista, CA, USA) at the Neuroscience Translational Research Solution Center (Busan, Korea).
E2 serum concentrations were determined in duplicate samples using E2 enzyme-linked immunosorbent assay (ELISA) kits (IBL) according to manufacturer’s instructions. The sensitivity of the E2 assay was 9.71 pg/mL, and the intraassay and interassay coefficients of variation were 2.7% and 7.2%, respectively.
RESULTS AND DISCUSSION
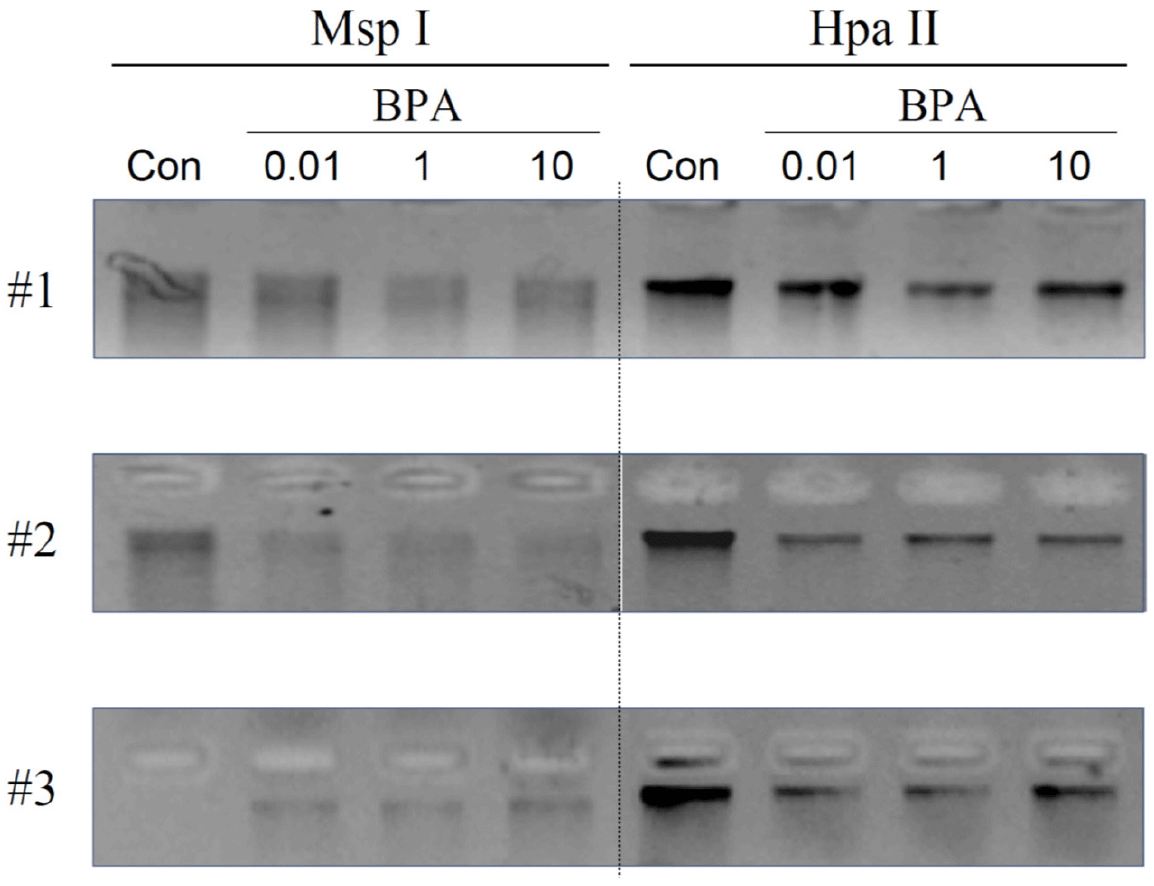
During the last 20 years, numerous reports have demonstrated the adverse effects of BPA on female reproduction, particularly fertility (Ziv-Gal & Flaws, 2016; Pollard et al., 2019; Pivonello et al., 2020). Among these, a limited number have investigated the effects of BPA exposure during pregnancy on biochemical and molecular changes in the uteri of the offspring born to exposed mothers. This is important because uterine disorders are directly linked to the failure of implantation and maintenance of pregnancy. More importantly, exposure to exogenous or environmental chemicals during the gestational period (particularly during the period encompassing gonadal sex determination and development) can provoke epigenetic alterations in germ cells, which can be transmitted to subsequent generations. Pregnant rats were exposed to three doses (0.01, 1, and 10 mg/kg BW) of BPA, and changes in global DNA methylation in uterine tissues obtained from adult offspring born to the exposed mothers were analyzed. Global DNA methylation analysis revealed that gestational exposure to BPA resulted in DNA hypomethylation in the uterus (Fig. 1). As shown in Fig. 1, the degree of methylation varied among individuals (animals) and/or treatments. This result indicates that several genes could be aberrantly upregulated because the corresponding promoters cannot be repressed by an altered DNA methylation status.
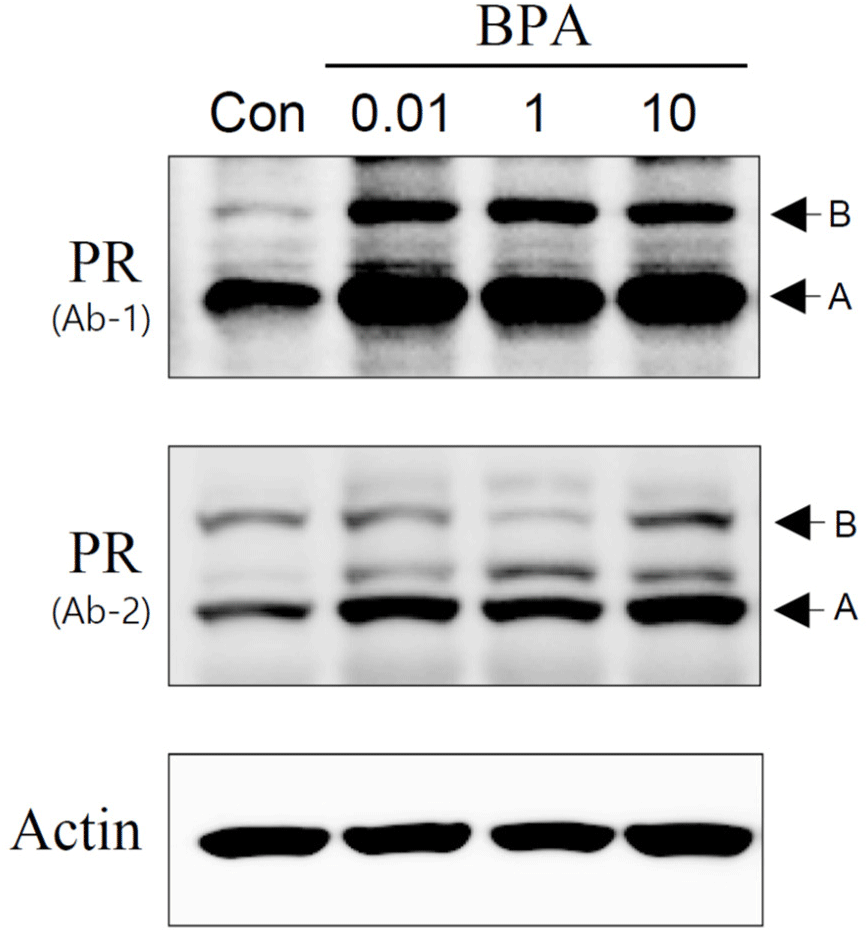
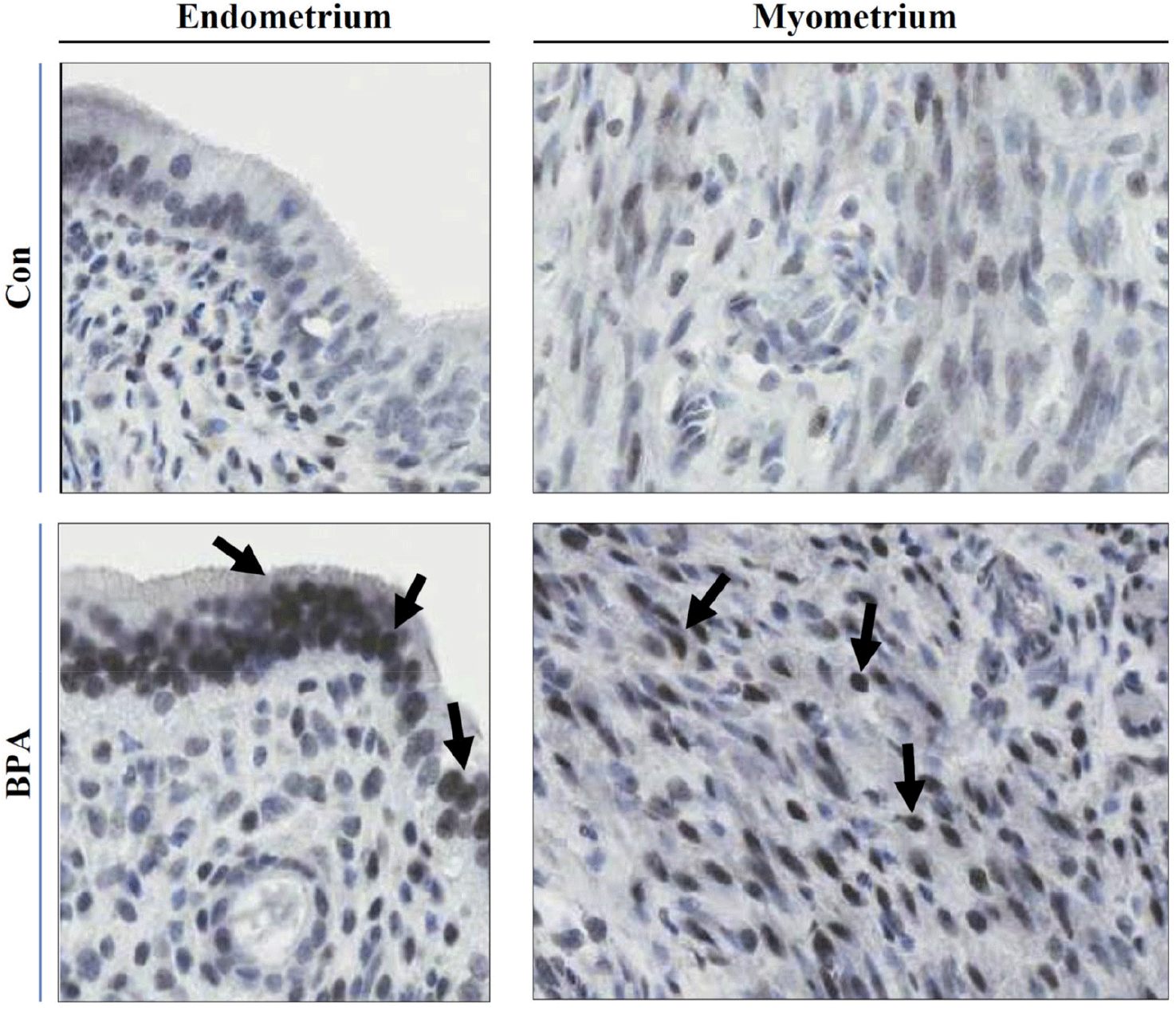
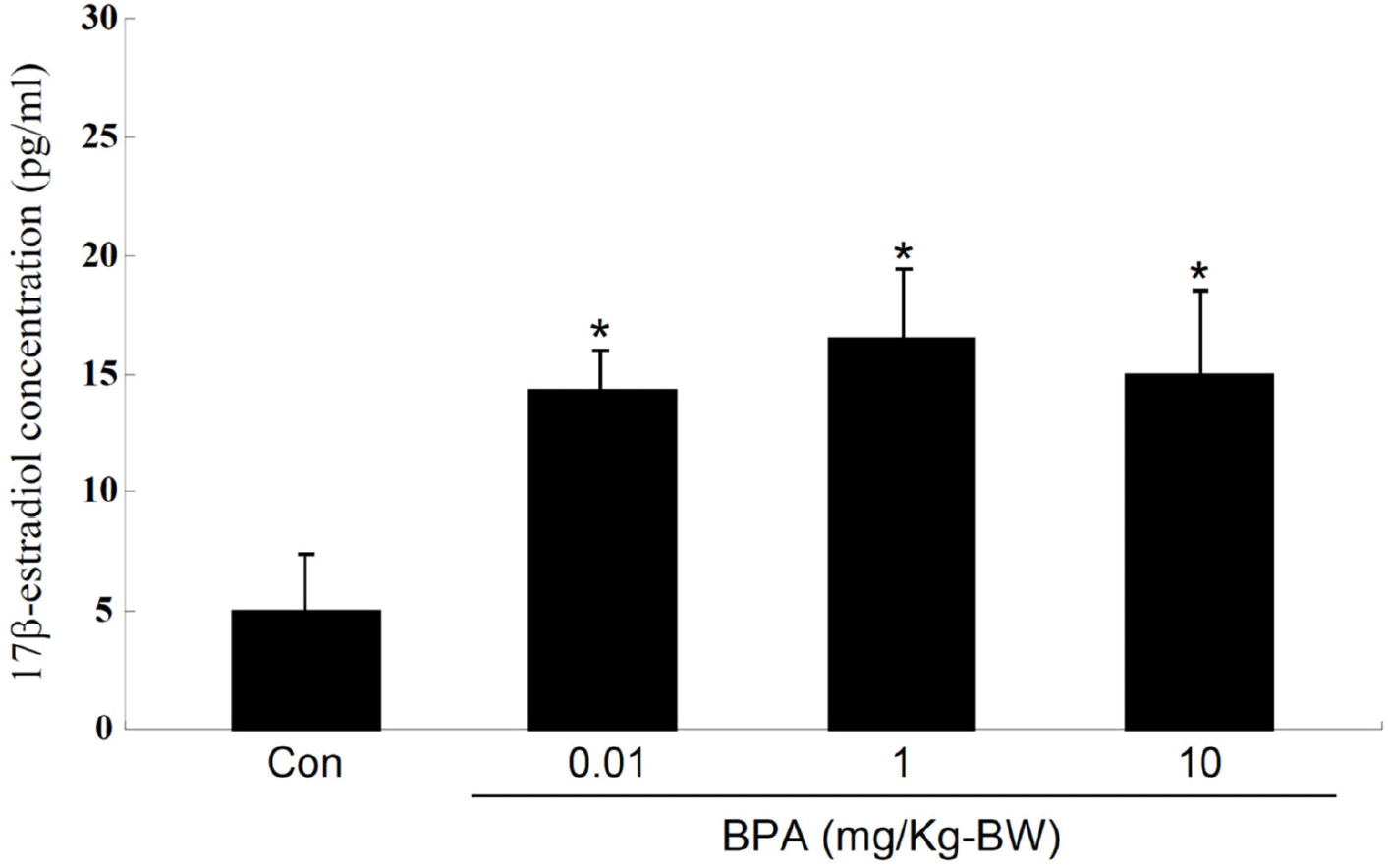
In this study, we investigated the status of the PR, a protein associated with the regulation of uterine function, expressed in F1 adult rats exposed to BPA during the gestational period. Although this approach appears to not be as systematic as a genome-wide methylation analysis, we first chose the PR as a candidate gene product of which a promoter could be potentially unmethylated. PR protein expression in uterine tissues was monitored using western blot analysis, which revealed that the PR protein content was considerably higher in all BPA-exposed groups than in the control (Fig. 2). These results were confirmed by employing two different types of PR antibodies (Fig. 2). Immunohistochemical examination for the PR revealed that intense PR-positive cells were more frequently observed in the BPA-exposed group than in the control group (Fig. 3). As shown in Fig. 3, the PRs were mainly localized in the nuclei of the endometrial and myometrial cells. PR expression is upregulated by 17β-estradiol (E2) secreted by the ovaries (Ing & Belen Tornesi, 1997; Meikle et al., 2000). Therefore, serum E2 levels were measured to determine the increase in PR expression in the uterus. E2 levels in all BPA-exposed groups were significantly higher than those in the control group (Fig. 4). Hence, we cannot exclude the possibility that the increased PR expression observed in the BPA-exposed groups may be due to the influence of increased E2. To date, the evidence that the upregulation of PRs observed in the present study was caused by the non-methylation of specific PR promoter regions is lacking. If necessary, this can be achieved using PR methylation-specific PCR in the near future.
Prenatal exposure (gestational day [GD] 9–GD 16) to BPA at environmentally relevant doses exhibits adverse effects on the uterui of mice later in life (Newbold et al., 2009). These include squamous metaplasia, atypical hyperplasia, and stromal polyps of the uterus. Perinatal exposure (GD 9–postnatal day [PND]21) to BPA increased the thickness of the uterine epithelia and stroma, reduced apoptosis in uterine epithelial cells during the estrus phase of the cycle, and downregulated estrogen receptor α expression in offspring mice (Adriana Mendoza-Rodríguez et al., 2011). BPA exposure in utero (GD 9–GD 16) provokes epigenetic alterations in the homeobox gene Hoxa10 in the uteri of F1 offspring (Smith & Taylor, 2007; Bromer et al., 2010). Increased Hoxa10 protein levels were observed in the uterine tissues of mice exposed to BPA in utero. As mentioned above, few studies investigated the effects of gestational BPA exposure on epigenetic alterations in genes involved in uterine function later in life. In addition, most studies employed different periods of exposure and different ranges of BPA doses. Despite these experimental inconsistencies, the common observation is that exposure to BPA during gestation provokes histological and genetic alterations in the uterus of an adult offspring. We believe that the time window of exposure (GD 8–GD 15) chosen in the present study would be particularly potent because most epigenetic alterations (e.g., remethylation) susceptible to gonadal sex determination and gonadal development have been accomplished.
Conclusively, these results indicate that exposure to BPA during gestation induces epigenetic alterations in the uteri of adult female offspring. We speculate that the global DNA hypomethylation and upregulation of the PR observed simultaneously in this study might be associated with the uterus. Therefore, it is important to elucidate the molecular evidence that PR gene expression is epigenetically altered in the uterus following BPA exposure in utero.







