INTRODUCTION
Ras-related (Rab) proteins, part of the monomeric G-protein family, play a crucial role in regulating intracellular vesicular transport. These proteins are predominately expressed in organelles and various transport vesicles (Pereira-Leal & Seabra, 2000; Wu et al., 2010; Goody et al., 2017). Functionally, Rab proteins are key players in membrane trafficking, including processes such as vesicle formation, vesicle movement, and membrane fusion (Alory & Balch, 2001; Stenmark, 2009; Hutagalung & Novick, 2011). Additionally, they contribute to the establishment and maintenance of the Golgi structure by controlling Golgi trafficking (Smith et al., 1990; Liu & Storrie, 2012; Goud et al., 2018).
Numerous studies have highlighted the importance of Rab proteins in male reproduction. They are implicated in the regulation of male meiosis (Shan & Sun, 2021), with Rab 12 displaying high expression levels in Sertoli cells (Iida et al., 2005) and Rab8B being associated with dynamic junctions in the testes (Lau & Mruk, 2003). During spermatogenesis, primordial sperm cells undergo structural changes, where the Golgi apparatus transforms into the acrosome in the sperm head (Nakamura et al., 2015; Kumar et al., 2016; Lin et al., 2017). Membrane trafficking involving Rab proteins is reported to play an important role in acrosome biogenesis, and Rab proteins are essential factors for the acrosome reaction and exocytosis (Moreno et al., 2000; Ramalho-Santos et al., 2001). In recent years, studies have investigated the involvement of Rab proteins in sperm motility. For example, Bae et al. demonstrated that various Rab proteins are present in the sperm head and tail, with several Rab proteins correlating with motion parameters (Bae et al., 2019, 2022a).
Rab3A plays a vital role in plasma membrane repair (Vieira, 2018) and is crucial for lymphocyte chemotaxis, regulating directional leukocyte motility (Constantin & Laudanna, 2010). It has also been linked to acrosomal formation and exocytosis in spermatozoa (Yunes et al., 2000). Furthermore, Rab3A exhibits correlations with specific sperm motility and motion kinematics, such as medium sperm motility and mean dance (Bae et al., 2022b). Although prior studies suggest an association between Rab3A and sperm motility, these findings are based on small population samples and provide limited information. Therefore, there is a need to identify additional sperm motility-related markers associated with Rab3A through more objective evaluations using larger sample sizes. The present study aims to evaluate the correlation between Rab3A and sperm motility parameters by assessing Rab3A expression levels and sperm kinematic characteristics in 150 boar spermatozoa. Additionally, statistical analysis was performed to estimate the correlations between Rab3A protein expression and sperm kinematic characteristics.
MATERIALS AND METHODS
Total 150 individual semen samples semen samples were collected from healthy mature Duroc boars (24–36 months old) using the gloved-hand technique. These samples were diluted with a broad extender [1:1 (v/v)] in Beltsville thawing solution (37 mg/mL glucose, 6 mg/mL sodium citrate, 1.25 mg/mL EDTA, 1.25 mg/mL sodium bicarbonate, and 0.75 mg/mL potassium chloride). The extended semen was stored in a low-temperature incubator (17°C) and processed within 2 h of collection (Jang et al., 2022).
Sperm motility and motion kinematic parameters were analyzed using a computer-assisted sperm analysis (CASA) program (IVOS® II, Hamilton Thorne, Beverly, MA, USA). Postincubation, 3 μL of the sample was placed on a preheated Makler counting chamber at 37°C (Sefi-Medical Instruments, Haifa, Israel). Measurements included total sperm motility (MOT, %), progressive sperm motility (PRG, %), curvilinear velocity (VCL, μm/s), straight-line velocity (VSL, μm/s), average path velocity (VAP, μm/s), mean amplitude of lateral head displacement (ALH, μm), beat-cross frequency (BCF, Hz), linearity (LIN, %), and straightness (STR, %).
Rab3A protein expression in spermatozoa was determined using an enzyme-linked immunosorbent assay. Spermatozoa were treated with rehydration buffer to extract total proteins [7 M urea, 2 M thiourea, 4% (w/v) 3-[(3-cholamidopropyl) dimethylammonio]-1-propane sulfonate, 1% (w/v) octyl b-D-glucopyranoside, 24 mM phenylmethylsulfonyl fluoride, 1% (w/v) dithiothreitol, 0.05% (v/v) Triton X-100, and 0.002% (w/v) bromophenol blue] at 4°C for 1 h. Protein concentration was measured following the Bradford protein-binding protocol (Bradford, 1976). Extracted protein (50 μg) was loaded into 96-well plates and incubated overnight at 4°C. The plates were then washed with PBS containing 0.05% Tween-20 (PBST) and blocked with a blocking solution [1% (w/v) bovine serum albumin in PBST] for 90 min at 37°C. Subsequently, the plates were incubated with anti-Rab3A antibody (1:5,000; Abcam, Cambridge, UK) for 90 min at 37°C, followed by incubation with goat anti-rabbit IgG H&L (HRP) antibody (1:5,000; Abcam) for 90 min at 37°C. Tetramethylbenzidine solution was used to activate peroxidase for 15 min at room temperature. Activation was terminated with 1 N sulfuric acid, and Rab3A protein signals were detected at 450 nm using a microplate reader (Gemini Em; Molecular Devices, Sunnyvale, CA, USA).
Data were analyzed using SPSS (ver. 26.0, IBM, Armonk, NY, USA). The correlation between Rab3A protein expression and sperm motility was analyzed using Pearson correlation coefficients. Student’s two-tailed t-test was used to compare sperm motility values. Data are presented as means±SEM, with statistical significance set at p<0.05.
RESULTS AND DISCUSSION
Rab proteins are integral to the intracellular vesicular transport system, playing a critical role in both preserving and establishing the Golgi apparatus through membrane trafficking regulation (Smith et al., 1990; Stenmark, 2009; Liu & Storrie, 2012). These proteins are also recognized for their involvement in male fertility, contributing to the origin of the acrosome from the Golgi apparatus during spermatogenesis (Moreno et al., 2000; Ramalho-Santos et al., 2001). Additionally, Rab proteins contribute to acrosome formation and reaction as well as sperm motility (Bae et al., 2022a). Specific Rab proteins, such as Rab8B, are associated with dynamic junctions in the testes (Lau & Mruk, 2003), and Rab2A serves as a biomarker for boar fertility evaluation (Kwon et al., 2015). Notably, Rab3A is a protein associated with acrosomal formation and exocytosis in spermatozoa (Yunes et al., 2000).
The fertility of mammalian spermatozoa is contingent of a specific duration within the female reproductive tract. During this period, sperm motility and motion kinematic parameters undergo changes with hyperactivation. Sperm motility is vital for navigating the tract and penetrating barriers, such as the zona pellucida (Jung et al., 2022). Furthermore, numerous studies have found a positive correlation between sperm motility and fertilization (Bongso et al., 1989; Donnelly et al., 1998), emphasizing the sperm motility’s pivotal role in successful fertilization. Specifically, Rab3A protein is correlated with medium sperm motility and mean dance (Bae et al., 2022b). However, for a more precise understanding, additional verification is essential to establish the correlation between Rab3A and sperm motility; therefore, the present study was performed to investigate this potential correlation.
In this study, the CASA program was used to estimate sperm motility and motion kinematics parameters. The analysis included average sperm motility, motion kinematic parameters, and Rab3A expression, yielding the following results: MOT = 88.0980% ± 0.4104%; PRG = 65.0233% ± 0.9996%; VAP = 99.5033 ± 0.8861 μm/s; VCL = 186.0728 ± 2.1710 μm/s; VSL = 65.5099 ± 1.1006 μm/s; ALH = 7.3627 ± 0.0915 μm; BCF = 36.2617 ± 0.2233 Hz; LIN = 37.0036% ± 0.7310%; STR = 65.5908% ± 0.8952%; and Rab3A = 0.0771 ± 0.0007 (Table 1; Fig. 1). Interestingly, Rab3A protein expression levels exhibited a positive correlation with several motion kinematic parameters. Specifically, Rab3A was positively correlated with VAP, the velocity over the average trajectory of the sperm cell (p<0.05; Table 2; Fig. 2A), ALH, the sperm head displacement along its curvilinear trajectory around the mean trajectory (p<0.05; Table 2; Fig. 2C), and VCL, the instantaneous velocity along the total trajectory of the sperm cell (p<0.01; Table 2; Fig. 2B) (Suarez & Dai, 1992; Rodríguez-Gil et al., 2007; Bae et al., 2022a). Several motion parameters, including VCL, ALH, linearity (LIN: VSL/VCL), and straightness (STR: VSL / VAP × 100), are used to categorize hyperactivation (Hinrichs & Loux, 2012). Hyperactivation indicates alterations in sperm motility, enabling penetration of the zona pellucida and fertilization of the oocyte. Notably, VCL, ALH, and LIN are associated with hyperactivated motility. Furthermore, sperm motion parameters, such as VCL, VSL, VAP, and ALH, have been linked to the outcomes of in vitro fertilization and intrauterine insemination (Aghazarian et al., 2021).
| MOT | PRG | VAP | VCL | VSL | ALH | BCF | LIN | STR | Rab3A |
|---|---|---|---|---|---|---|---|---|---|
| 88.0980±0.4104 | 65.0233±0.9996 | 99.5033±0.8861 | 186.0728±2.1710 | 65.5099±1.1006 | 7.3627±0.0915 | 36.2617±0.2233 | 37.0036±0.7310 | 65.5908±0.8952 | 0.0771±0.0007 |
Sperm motility and motion kinematic parameters are presented as means±SEM. n=3.
MOT, total sperm motility (%); PRG, progressive sperm motility (%); VAP, average path velocity (μm/s); VCL, curvilinear velocity (μm/s); VSL, straight-line velocity (μm/s); ALH, mean amplitude of head lateral displacement (μm); BCF, beat-cross frequency (Hz); LIN, linearity [%, (VSL / VCL) × 100]; STR, straightness [%, (VSL / VAP) × 100].
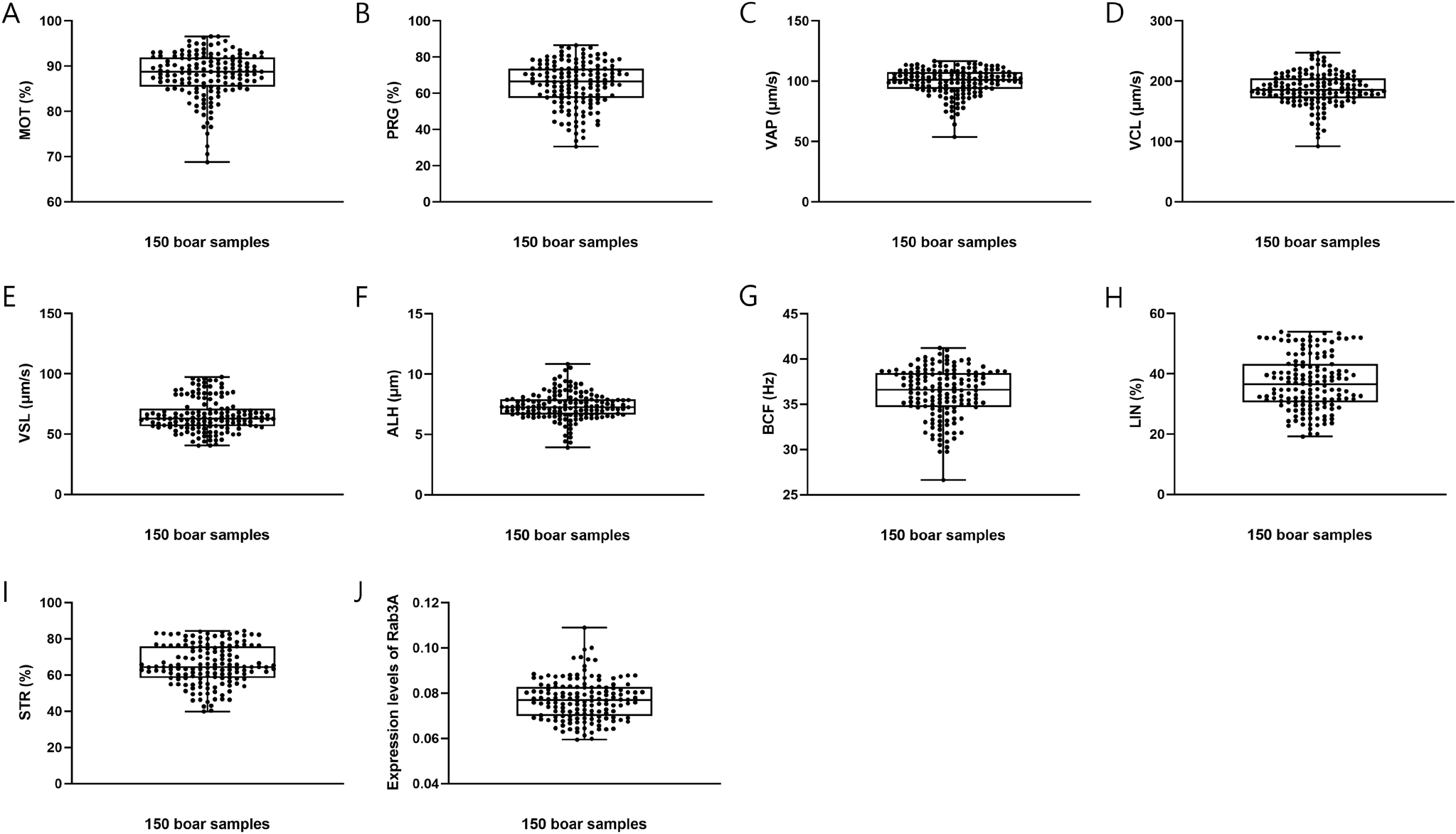
MOT, total sperm motility (%); PRG, progressive sperm motility (%); VAP, average path velocity (μm/s); VCL, curvilinear velocity (μm/s); VSL, straight-line velocity (μm/s); ALH, mean amplitude of head lateral displacement (μm); BCF, beat-cross frequency (Hz); LIN, linearity [%, (VSL / VCL) × 100]; STR, straightness [%, (VSL / VAP) × 100].
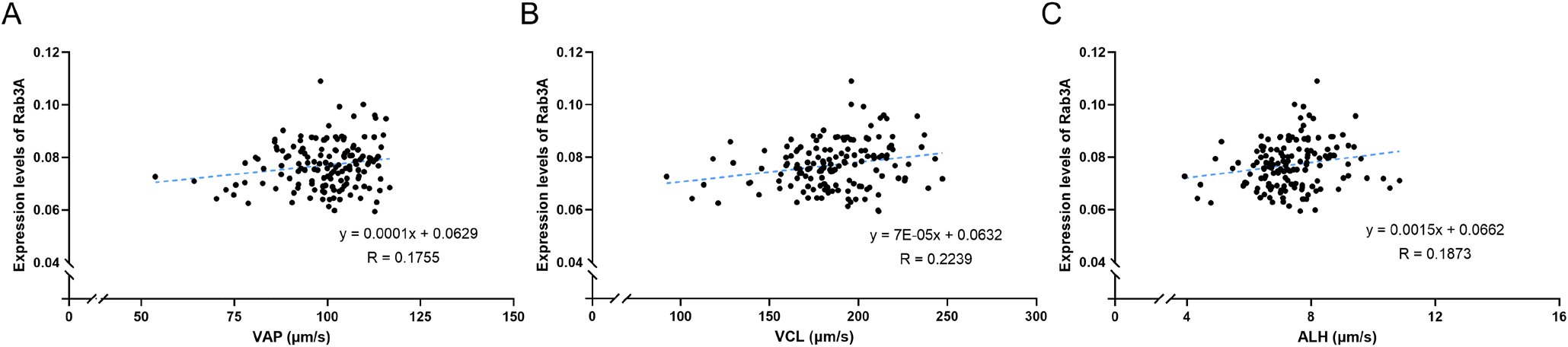
In a prior study, Rab3A was associated with litter size; consequently, it can serve as a biomarker for predicting fertility (Bae et al., 2022b). In the present study, Rab3A was associated with various motion parameters, suggesting the protein’s important role in motility-related fertility. Additionally, based on previous research (Bae et al., 2022b), Rab3A expression is correlated with medium sperm motility and mean dance (mean amplitude of head lateral displacement / linearity). Although prior studies on Rab3A primarily focused on acrosome formation and exocytosis, our results demonstrate its associated with various motion parameters. Thus, it is proposed that the Rab3A protein plays a crucial role in male fertility through its correlation with sperm kinematic characteristics. Overall, the data from this study can serve as foundational information for further exploration of the relationship between Rab proteins and sperm motility.







