INTRODUCTION
The developmental ability of oocyte is dependent on various factors including size, accumulation of nutrients and other factors including developmental determinant factors. To get the developmental potency, the growing oocytes should be stay in GV intact status, prophase I of meiosis and growth to a species-specific size. The meiotic cell cycle in mammalian oocytes proceeds up to the dictiate stage of the first prophase. Although dictiate stage arrest (GV arrest) is critical in gametogenesis, only few of cellular molecules are known to be involved in GV arrest during oogenesis.
A few of cellular molecules are known to be involved in GV arrest during oogenesis. In vitro condition, spontaneous maturation of denuded oocyte (DO) and cumulusenclosed oocyte (CEO) can be inhibited or delayed by cyclic adenosine monophosphate (cAMP) which are injection of 3’,5’-adenosine monophosphate (Tsafriri et al., 1972), folskolin induced activation of adenylate cyclase (Ekholm, 1984), follicle stimulating hormone (FSH, Eppig et al., 1983), etc.. Co-treatment of FSH and adenosine (1–30 μM) markedly inhibits the germinal vesicle breakdown (GVB) of CEOs in a dose-dependent manner (Miller & Behrman, 1986).
The spontaneous resumption of meiosis after removed from the intrafolliclular environment is not affected by the concentration of external calcium in the absence of dibutyryl cyclic AMP (dbcAMP): GVB was unaffected by the calcium ionophore A23187, the calcium antagonists verapamil or tetracaine, and calcium-depleted medium (Paleos & Powers, 1981). However, in the presence of FSH, adenosine inhibits calcium-induced oocyte maturation in CEO but those have no effects on that in denuded oocytes (Preston et al., 1987). Therefore it has been suggested that the action of dbcAMP is mediated by its effect upon this calcium (Powers & Paleos, 1982). The activation of MPF is necessary for store-operated Ca2+ entry (SOCE) inactivation during oocyte maturation (Machaca & Haun, 2002). On the other hand, inositol 1,4,5-triphosphate (IP3) receptor sensitivity is increased at the time of MPF activation and IP3-dependent Ca2+ release needed for MPF activation (Sun et al., 2009). Calmodulin antagonist inhibit GVB (Carroll & Eckberg, 1986).
On the other hand, a few of molecules were studied to explain the long time GV arrest during oocyte growth. Early mitotic inhibitor 1 (Emi1)-dependent regulation of APCcdh1 is essential for regulating prophase I arrest. The destruction of Emi1 delays entry into meiosis 1 and prevents normal M1 spindle formation in mouse oocyte (Marangos et al, 2007; Reis et al., 2006). APC activity that is involved in inactivation of MPF is modulated by PIK and PKA (Kotani et al., 1998). A-kinase anchoring proteins (AKAPs) mediate the intracellular localization of PKA and control the specificity and kinetics of substrate phosphorylation. APAP/PKA interactions maintain the GV arrest (Newhall et al., 2006). Natriuretic peptide precursor type C (NPPC) acting via natriuretic peptide receptor 2 (nPR2) maintain meiotic arrest (Zhang & Xia, 2012).
The synergism of adenosine with forskolin on meiotic arrest does not require uptake of the nucleoside nor its conversion to ATP and that adenosine effects are exerted at the level of the oocyte plasma membrane (Salustri et al., 1988). The meiotic blocking of hypoxanthine plus adenosine is reversed by uptake the adenosine in denuded oocyte and it is suggested that the action of adenosine is not due to hypoxanthine salvage pathway (Downs, 1999). In addition, the putative metabolite cAMP plays an important role in controlling meiotic arrest of oocytes in most species studied. Elevated cyclic AMP levels in defolliculated oocytes to the same extent as in follicular oocytes. However, it is not enough to explain the complex results of adenosine in meiotic arrest.
Purine can work in the cells which express its receptors. Adenosine can work through its receptors on plasma membrane. Adenosine receptor has four subtypes, A1, A2A, A2B and A3. All of these subtypes belong to the superfamily of G protein-coupled receptors (GPCRs), with the A1 and A3 adenosine receptors interacting with pertussis toxin (PTX)-sensitive G proteins, including Gi and Go (Gi/o) proteins, and the A2A and A2B adenosine receptors interacting with Gs protein (Epperson et al., 2009). It means that adenosine can induce various cellular signaling pathways through its receptors.
Although adenosine involve in blocking of meioitic division depending on the conditions, the mechanisms are not clear. Based on them it is hypothesized that adenosine may involve in GV-arrest through adenosine receptors of cumulus cells. Therefore in this study, we investigate that adenosine has interaction with cumulus during oocyte meiotic arrest and that express adenosine receptor aspect in cumulus enclosed oocyte.
MATERIAL METHOD
All experiments involving animals were conducted in accordance with the National Institutes of Health standards for the use and care of animals. The animal protocols were approved by the Sungshin Women’s University Institutional Animal Care and Use Committee. CD-1 mice were maintained on a 14 h light and 10 h dark cycle under standard vivarium conditions, and supplied with food and water ad libitum. Immature female mice (3 weeks old) were injected with 5 units of gonadotropin from pregnant mares’ serum (PMSG) to enhance multiple follicular developments. The mice were sacrificed by cervical dislocation at 46 h post PMSG injection. Then their ovaries were removed and transferred to BWW medium (94.6 mM NaCl, 4.78 mM KCl, 1.19 mM KH2PO4, 1.19 mM MgSO4 • 7H2O, 1.71 mM calcium lactate, 21.58 mM sodium lactate, 0.3 mM sodium pyruvate, 25.07 mM NaHCO3, 100 units/ml penicillin, 100 μg/ml streptomycin, pH 7.4, and 0.4% BSA). Oocytes were collected by ovarian follicular puncture with needle under a dissecting microscope and collected the CEO. All this procedure did within 20 minutes for escape spontaneous maturation. Only healthy immature oocytes were chosen for culture.
Oocytes were cultured in 10 μl drops of BWW medium under the mineral oil (Sigma) for 18 h at 37°C in a humidified CO2-controlled (5%) incubator. To study the effects of the adenosine on oocyte maturation, immature oocytes were cultured in BWW contained 50 or 750 μM adenosine. It is known that the concentration of adenosine is 750 μM in follicular fluid. The maturation stages were scored with a differential interference contrast microscope (Carl Zeiss, Germany).
cDNA systhesis was used sidestep™ QPCR cDNA synthesis Kit. For A1, the forward and reverse primers were 5’-GTCTCTGTGCCCGGAAATGTAC-3’ and 5’-CGTGTGTGAGGAAGATGGCAAT-3’3’, which amplified a fragment of . For A2a, the forward and reverse primers were 5’-GCAACCTGCAGAACGTCACAA-3’ and 5’-TC TGTCTGACTGCAGTTGTTCCAG-3’. For A2b, the forward and reverse primers were 5’-TGTCGACCCGATA TCTGCCAT-3’ and 5’-CATCAGTTCCATGCGCTGAAG -3’. For A3, the forward and reverse primers were 5’-TCTGAGGACCACCACCGTCTATTT-3’ and 5’-GGAT CCAGGTGATGAAGCTGAAA-3’.
Intracytoplasmic free calcium levels were monitored by a laser scanning confocal microscope. Follicular puncture did in the BWW medium containing 5 μM Fluo 3-AM and the CEO were further incubated for 15 min in the medium containing 5 μM Fluo 3-AM. After then, the CEO was washed five times with plane medium. The oocytes were seized between slide glass and coverslip using dot-slide and scanned every 2.1 s at 37°C under the laser scanning confocal microscope (Olympus, Fluo View). The results were expressed as the relative fluorescence intensity (RFI).
RESULT
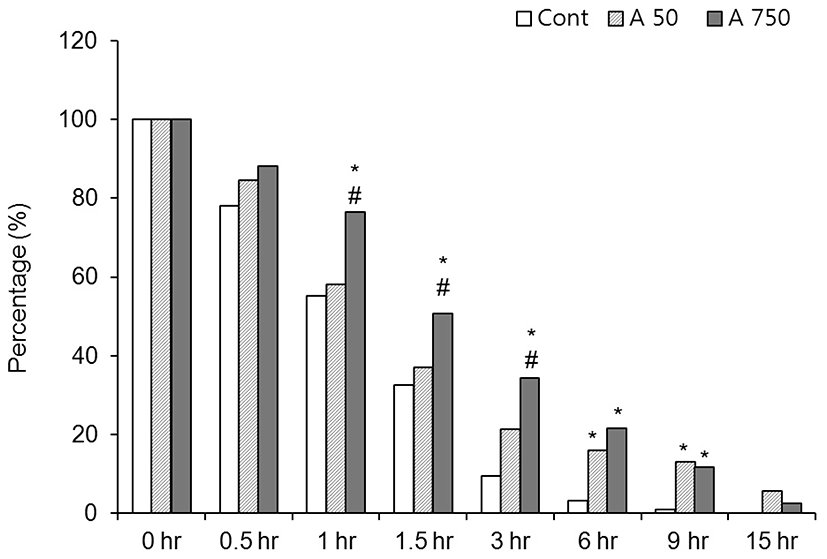
In control medium, 95% of the CEOs spontaneously released from GV-intact condition within 6 hr after release from the preovulatory follicle (Fig. 1). In the media containing 50 μM adenosine, the rate of GV-intact oocytes was increased but there was no statistical significance compared with the control until 1.5 hr after culture. After then, the rates of GV-intact were significantly high at 3 hr (9.4% vs 21.4%), 6 hr (3.1% vs 15.9%) and 9 hr (0.8% vs 11.6%) compared with the control. In 750 μM adenosine treated group, the arresting is kept longer than 50 μM group. The rates of GV-intact oocytes were significantly high compared with the control until 9 hr after culture. In 750 μM group, the rates of GV-intact were significantly high at 1 hr (58.1% vs 76.5%), 1.5 hr (37% vs 50.7%) and 3 hr (21.4% vs 34.3%), but after then there was no significance compared with those of 50 μM adenosine group (Fig. 1). Those data means GV-arresting effect of adenosine is lasting until 9 hr and it is direct proportional to the concentration of adenosine.
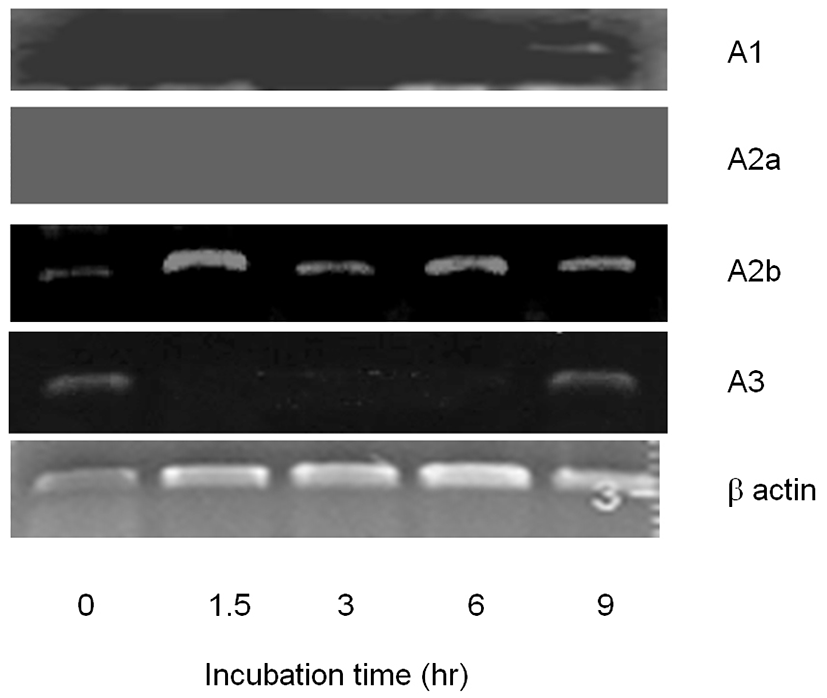
To evaluate the working pathway of adenosine, we tried to identify the existence of adenosine receptor(s) in CEO. A1 receptor was detected in CEO at 9 hr. A2b also detected in CEO in all time points (Fig. 2). These results explain that A1 and A2b receptors are expressed in the cumulus and those are spatio-temporally expressed 9 hr after released from follicle. In the case of A2a, it was not detected in all the groups. A3 was detected in CEO at 0 hr and 9 hr time points after released from follicles (Fig. 2). It means that cumulus cell expresse A3 in a time-dependent manner.
A1 activation promotes an increase on alpha(2)-adrenoreceptor binding in brainstem cell through phospholipase C, protein kinase Ca2+-dependent, IP3 receptor and intracellular calcium (Carrettiero et al., 2009). A2bR couple to G(s)-adenylyl cyclase and its agonist elevates cellular cAMP and stimulates extracellular signal-regulated kinase 1/2 activity (Epperson et al., 2009). In nucleus tractus solitary (NTs), phospholipase C, protein kinase Ca2+-dependent, IP3 receptor and intracellular calcium is participate in A2aR and A1R interaction (Carrettiero et al., 2009). A3 does not induce the calcium uptake in the mitochondria (Xia et al, 2007) but it involved in [Ca2+]i fluctuation in BON cell. From them we know that calcium is indirectly involved in signaling of adenosine receptors.
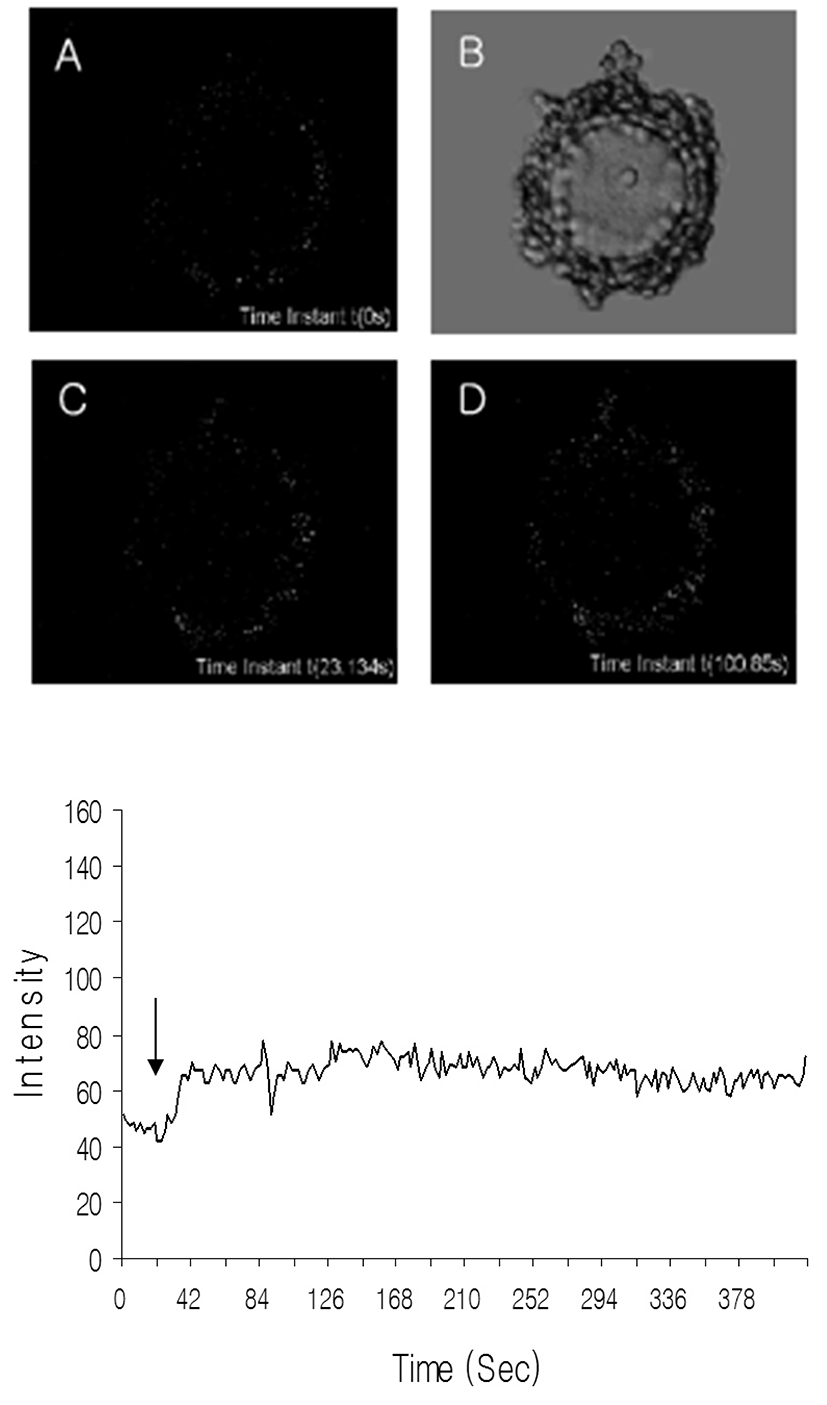
To know whether the adenosine can work with calcium in GV arrest, the level of free calcium was measured as mentioned in Materials and Methods. Calcium levels were increased by adenosine treatment in cumulus cell within a few seconds as shown in Fig. 3. In the case of oocytes, the levels of calcium were not changed dramatically and its small fluctuations appeared about 70 seconds after adenosine treatment. These results suggest that A3 may regulate the calcium levels directly in cumulus cell and indirectly in oocytes. Also, those results revealed that calcium is not direct regulator of GV arrest.
DISCUSSION
Adenosine is an important regulator of cellular function, being released by numerous cells under a variety of conditions to act in paracrine or autocrine capacity. It can induce cellular response through its membrane receptors. All the adenosine receptor subtypes belong to the superfamily of G protein-coupled receptors (GPCRs) (Kobayashi et al., 2002). Affinity to the adenosine of these receptors is difference between them and the effects of adenosine depend on their concentration. In here we showed that adenosine delay GVB and keep GV-intact during a limited time.
So far the roles of adenosine in meiotic arrest are controversy. One of the possible roles is adenosine potentiates the meiotic arrest induced by FSH in CEO (Downs, 1999). Downs and Chen (2006) reported that native adenosine has meiosis-inducing capability. When tested various culture conditions, meiotic induction by 8-bromo-adenosine (8-Br-Ado) and a second adenosine analog, methyl-mercaptopurine riboside (MMPR), was especially potent in denuded oocytes (D) compared to CEO and was not dependent on the type of inhibitor chosen to maintain meiotic arrest. Adesnosine and inhibitors of de novo purine synthesis had no effect on the completion of maturation (Downs & Chen, 2006). It also known that the concentration of purines in follicular fluid are not same between follicular stages. Interestingly, in our results, we could get following results; adenosine bolocked the GVB in a concentration dependent manners in a restricted period in in vitro. In the case of adenosine treated groups, the rates of GV-arrest was significantly higher in CEO groups until 9 hr post in vitro culture. GV-arresting effect of adenosine is lasting until 9 hr and is direct proportional to the concentration of adenosine. It is revealed that during folliculogenesis the adenosine can inhibit GVB through cumulus cells and that adenosine may work through cumulus cells to GV arrest in higher concentration.
Adenosine treatment together with forskolin produces a further delay in the resumption of meiosis. Dipyridamole, which inhibits adenosine transport, does not prevent the meiosis-arresting synergistic effect of adenosine with forskolin (Salustri et al., 1988). As seen the results, adenosine delay the resumption of meiosis. In addition, during in vitro maturation there adenosine receptors expressed time dependently. These mean that adenosine works through its receptors on the cumulus cells to delay the meiotic resumption.
The follicular fluid contains the purines, 2-4 mM hypoxanthine and 0.34-0.78mM adenosine, and that the concentrations of these purines decrease immediately before gonadotropin-induced germinal vescle breakdown (Eppig et al., 1985). In the case of porcine, follicular fluid contains hypoxanthine (1.4 mM), adenosine (0.06 mM), uracil (0.44 mM), and 7-methyliosine (0.19 mM). The hypoxanthine contribute significantly to the maturationarresting activity of porcine follicular fluid (Downs et al., 1985). Put together with our results, it is suggested that adenosine in the follicular fluid may control the meiotic resumption through its receptors on cumulus cells.
So far, calcium is known as a meiosis regulator for prophase 1 arrest in oocyte. Increase the free calcium in oocyte is needed in activation of MPF (Machaca and Haun, 2002; Powers and Paleos, 1982; Sun et al., 2009). In the cumulus cells, however, the role of calcium in meiotic arrest is not much known. In here we revealed that the free calcium levels were increased by exposing to adenosine. Put together with the suppression effects on meiotic resumption of adenosine, this result means that the calcium increase in cumulus cells involved the regulation of meiotic arrest.
At 1988 Salustri and his colleagues suggested that adenosine effects are exerted at the level of the oocyte plasma membrane. In here we showed that adenosine also suppress meiotic resumption through it receptors on the cumulus cells. In addition, it is suggested that the increased calcium levels in cumulus by adenosine may involve in the meiotic regulation by cumulus cells, even though the further studies are needed.