INTRODUCTION
During embryogenesis, developmental regulators, such as growth factors, transcription factors, and RNA-binding proteins, control the processes of growth, differentiation, and morphogenesis, which shape and pattern to the growing embryo. Many studies have focused on the role of RNA-binding proteins in growth, differentiation, and morphogenesis in vertebrates (Tanaka et al., 1997; Wakamatsu & Weston, 1997; Wu et al., 1997; Peng et al., 2000; Gerber et al., 2002).
Fused in sarcoma (Fus), also known as translocated in liposarcoma (TLS), POMP75, and pigpen, is a nuclear DNA/RNA-binding protein that regulates the different steps of gene expression, including transcription, splicing, and mRNA transport (Dormann et al., 2013). FUS not only regulates gene expression in the nucleus but also exerts important functions in the cytoplasm. FUS has been suggested to continuously shuttle between the nucleus and cytoplasm (Zinszner et al., 1997).
The N-terminus of FUS appears to be involved in transcriptional activation, while the C-terminus is involved in protein and RNA binding. In addition, recognition sites for the transcription factors AP2, GCF, and Sp1 have been identified in FUS (Aman et al., 1996). FUS/TLS is a member of the TET protein family, which also includes EWS, the TATA-binding protein (TBP)-associated factor TAFII68/TAF15 and the Drosophila protein cabeza/SARF (Morohoshi et al., 1998; Bertolotti et al., 1999). Fus was identified approximately 20 years ago as a fusion oncogene in human myxoid liposarcoma (Crozat et al., 1993; Rabbitts et al., 1993). In this cancer, an aberrant chromosomal translocation fuses the N-terminus of FUS to a transcription factor, such as CHOP, giving rise to a fusion oncogene. Fus has been examined in various studies of neurodegeneration; mutations in FUS were reported to cause familial ALS, and FUS was found to be deposited in pathological protein inclusions (Kwiatowski et al., 2009; Vance et al., 2009).
Based on knockout studies, mice homozygous for the Fus mutation fail to suckle and die within 16 hours of birth. Fus–/– mice appear to develop normally, and histologic examination of serial sections confirms the normal structure and development of all major organs and tissues in these animals. However, the thymus is reduced in size in Fus–/– embryos, and the peripheral blood of newborn Fus–/– mice shows a reduced number of white blood cells. In addition, Fus–/– mice have diminished cellularity in their lymphoid organs (Hicks et al., 2000). Furthermore, bovine pigpen (Fus) has been suggested to be involved endothelial cell proliferation and differentiation (Alliegro & Alliegro, 1996). Pigpen mRNA and protein are expressed in endothelial cells. In a functional study, the nuclear injection of anti-pigpen antibodies was found to block endothelial cell division (Alliegro & Alliegro, 2002), and this study suggested that pigpen may play a role in regulating endothelial cell growth and differentiation.
Teeth are one of the most diverse organs in vertebrates, both morphologically and functionally. An early signaling event during tooth development is the induction of the mesenchyme by bone morphogenic protein (BMP) and fibroblast growth factor (FGF) from the epithelium. BMP and FGF induce the expression of several mesenchymal transcription factors, many of which are necessary for sequential tooth development (Thesleff et al., 2000). Thus, teeth provide an excellent model to study vertebrate organogenesis. Furthermore, tooth root development is also regulated by reciprocal interactions between epithelial and mesenchymal tissues, such as in crown development (Thesleff & Sharpe, 1997; Thesleff, 2000; Zhang et al., 2000;). After the crown formation stage, the Hertwig’s epithelial root sheath (HERS) is a proliferated epithelial cell at the cervical loop of the enamel organ in the developing tooth (Hosoya et al., 2009). It is composed of an epithelial bilayer derived from the inner and outer enamel epithelium that fuses below the level of the cervical loop of the crown. Although a considerable amount of data exists on the expression of many genes during tooth development, the precise gene expression pattern of Fus mRNA during tooth development has not been extensively studied, especially during tooth root development. In this study, we analyzed the expression pattern of Fus mRNA during tooth development by in situ hybridization.
MATERIALS AND METHODS
All experiments were performed according to the guidelines of the Yonsei University, College of Dentistry, Intramural Animal Use and Care Committee.
Adult ICR mice were housed in a temperature-controlled room (22°C) under artificial illumination (lights on from 05:00 to 17:00) at a relative humidity of 55% with access to food and water ad libitum. Embryos were obtained from time-mated pregnant mice. First mandible molar tooth germs of ICR mice at 11 dpc (days postcoitum), 12 dpc, 14 dpc, 16 dpc, postnatal day 4 (PN4), and PN14 were used for in situ hybridization and hematoxylin and eosin (HE) staining. Briefly, mandibles from each stage were fixed with 4% paraformaldehyde (PFA) in 0.01 M phosphate- buffered saline (PBS, pH 7.4) overnight at 4°C. After embedding the samples in paraffin, they were sectioned at a thickness of 5 μm.
In situ hybridization of the mouse mandible sections was performed using standard protocols (Eblaghie et al., 2004). The sections were baked at 65°C, de-waxed in xylene, rehydrated through a graded series of alcohol washes and post-fixed in 4% PFA. The sections were prehybridized in a humid chamber containing 50% formamide in 2×SSC at 55°C for 30 min. Digoxigenin (DIG)-labeled RNA probes were pre-warmed at 85°C and hybridized to the sections overnight at 65°C. We also performed negative staining using a sense probe and positive reactions did not appear (data not shown).
RESULTS
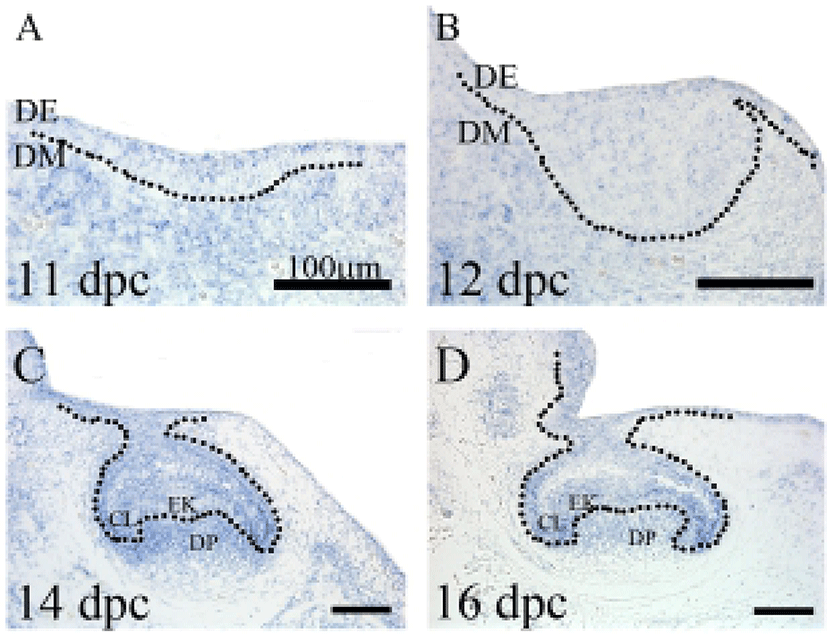
In the developing embryo, Fus was found to be highly expressed during early development, when all cells are rapidly proliferating and when expression becomes more restricted as the tissues differentiate (Alappat et al., 2003). To identify the spatial pattern of Fus expression, in situ hybridization was performed on serially sectioned embryonic teeth (from 11 dpc to 16 dpc). Fus-specific expression was detected in the developing tooth.
During early tooth development (at 11 dpc), Fus was expressed in the dental lamina and the presumptive dental mesenchyme of the developing tooth (Fig. 1A). Furthermore, Fus was distributed in the dental epithelium and dental mesenchyme (Fig. 1B). Interestingly, at the beginning of the cap stage (14 dpc), Fus showed strong expression in the cervical loops of the dental epithelium, where a high level of cell proliferation continues to occur, as well as in the subjacent dental mesenchyme. On the other hand, weak expression was detected in the enamel knot, which represents a subset of epithelial cells that have escaped the cell cycle (Fig. 1C). At 16 dpc, Fus expression was maintained strongly in the dental papilla and the cervical loops (Fig. 1D). The observed expression pattern of Fus in the early tooth germ is consistent with a previous study (Alappat et al., 2003).
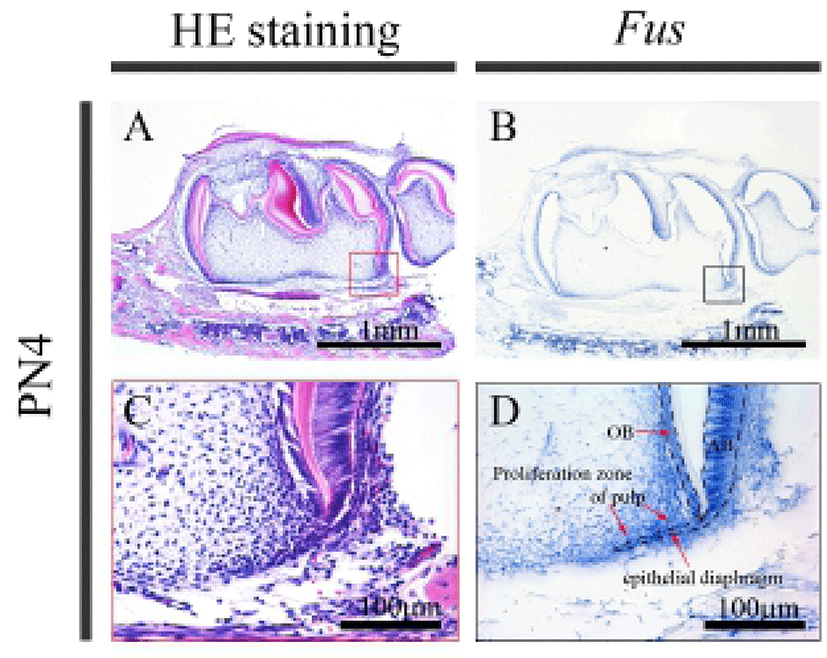
In order to understand postnatal molar tooth development in vivo, the histological appearance of sagittal sections of the mouse mandible at PN4 and PN14 was observed by HE staining. At PN4, odontoblasts and ameloblasts were present in the crown (Fig. 2A and C). In addition, the cervical loops were found in the apical region. At this stage, the HERS, the bilayered structure derived from the inner and outer enamel epithelium that fuses below the level of the cervical loops of the crown, is not yet developed. At PN4, Fus mRNA was expressed in the ameloblasts, not only around the cusp tip but also in the cervical loop (Fig. 2B and D). Fus was also expressed in the odontoblasts, the proliferation zone of pulp and the epithelial diaphragm.
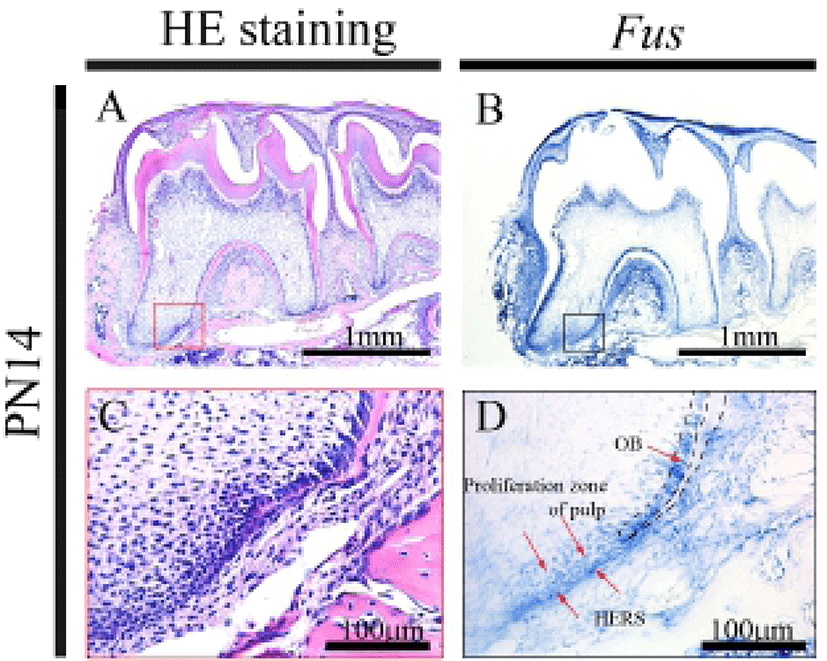
At PN14, the first, second, and third molars showed different stages of root development. The crowns of the first and second molars were almost fully developed, and the roots of these molars were developing (Fig. 3A and C). At the apical end of the growing root, the HERS was detectable. At this stage, the odontoblasts maintained the expression of Fus (Fig. 3B and D). In addition, Fus expression was observed in the proliferation zone of the dental pulp and remained strongly in the HERS.
DISCUSSIONS
Many genes are involved in normal tooth development, and mutational analyses of these genes have revealed how tooth development is regulated (Thesleff et al., 1997).
FUS, a RNA/DNA binding protein, has been suggested to play a role in regulating cell proliferation and differentiation in the neovasculature of developing tumors in the rat brain and in endothelial cells (Blank et al., 2001; Alliegro & Alliegro, 2002). Due to their complex nature, teeth are precisely controlled by congruent genetic and environmental regulation. Not much is known about Fus, not only with respect to its expression pattern but also its function and its role in normal tooth development. Therefore, using mice as a model, the goal of this study was to determine the expression pattern of Fus during the embryonic and postnatal stages of tooth development. Our results showed that Fus is expressed in the tooth bud from the initiation of tooth development (11 dpc). It is expressed in both the dental epithelium and the dental mesenchyme and is expressed particularly strongly in the cervical loops of the dental epithelium from the cap stage (14 dpc). Thus, the expression pattern of Fus correlates with early tooth development.
The genetic relationship in tooth root development has been examined (Hosoya et al., 2008). However, the relationship between Fus and other genes in root development has not yet been extensively assessed. Here, we examined the expression pattern of Fus at the apical end of the tooth germ during root development. Fus was detected in the odontoblasts and ameloblasts at PN4. Fus expression was maintained in the odontoblasts, dental pulp, and especially in the HERS at PN14. Therefore, the expression pattern of Fus indicates that this gene may be involved in the HERS during root development. Further studies are required to examine the function and role of Fus in root development. Moreover, its relationship with other genes should be studied with respect to this process.