INTRODUCTION
Oocyte developmental process in teleosts is under the influence of sex steroid hormone that is generated from the follicle layer, and the sex hormone is regulated by the pituitary gonadotropin (GtH) (Nagahama et al., 1994; Planas & Swanson, 2008). In female fish, estradiol-17β (E2), testosterone (T) and progestins are the main gonadal steroids. During vitellogenesis, E2 is the major sex steroid, synthesized at follicle cell layer and transported to liver. Then stimulated liver produced vitellogenin, the precursor of yolk protein. After vitellogenesis, progestins such as 17α,20β-dihydroxy-4-pregnen-3-one (17α20βP) and/or 17α,20β,21-trihydroxy-4-pregnen-3-one (17α20β21P) act as maturation inducing steroid (MIS) and induce final oocyte maturation including germinal vesicle breakdown (GVBD) and ovulation (Patiño & Sullivan, 2002; Baek, 2008; Nagahama & Yamashita, 2008). Androgens, T and 11-ketotestosterone (11-KT), are generally considered playing a role in regulating testis differentiation and spermatogenesis (Borg, 1994). However, it was reported in several species of fish including those of the Anguillidae family, that 11-KT was also identified as the major androgens in the blood of females (Lokman et al., 2002; Setiawan et al., 2012). The role of these sex steroids in fish reproductive process is still unclear, due to the high diversity of reproductive strategies. This may be different in fish species with synchronous or asynchronous ovarian development (Tyler & Sumpter, 1996).
The starry flounder (Platichthys stellatus), which is a regional specific species in East Sea of Korea, exhibits multiple spawning and asynchronous ovarian development (Lim et al., 2007; Hwang et al., 2012). Since it is getting hard to catching the natural starry flounder, we are planning to recover fisheries resource and increase fisheries stocks by releasing artificial fish seed; however, few studies have investigated concerning its reproductive characteristics. Understanding steroidogenesis in starry flounder could elucidate the endocrine control of reproductive cycle. Therefore, in this study, we analyzed in vitro oocyte steroidogenesis and identified steroid metabolites from vitellogenic and post-vitellogenic stages (mature oocytes) of development in the starry flounder.
MATERIALS AND MATHODS
The starry flounder used in this study were captured in coastal waters of Pohang, Korea during the breeding season (December-March). Oocytes were separated into groups using fine forceps. Oocytes with average diameters of 0.52-0.71 mm were used for incubation. After separating the ovaries into small pieces in ice-cold balanced salt solution (132.96 mM NaCl, 3.09 mM KCl, 0.28 mM MgSO4 • 7H2O, 0.98 mM MgCl2 • 6H2O, 3.40 mM CaCl2 • 6H2O, and 3.65 mM HEPES), approximately 30 follicle-enclosed oocytes were incubated in the presence of 55 kBq of [3H]-17αOHP as the radiolabeled precursor in each well of 24-well culture plates containing 1 ml of Leibovitz L15 medium (Gibco). The pH and osmolarity of the media were adjusted to 7.9 and 300 mOsm, respectively. The plates were incubated for 24 h at 12°C with constant gentle shaking. Some pieces of ovary from each individual were fixed in Bouin’s solution for 24 hours for histological observations of oocytes. The fixed samples were washed, dehydrated, and embedded in paraffin. Serial sections of 4-6 μm thickness were prepared; the slides were stained in Mayer's hematoxylin and 0.5% eosin and mounted with malinol. Histological samples were observed through a light microscope (BX50, Olympus, Japan).
At the end of the incubation, steroids were extracted three times from the media and oocytes using 4 ml dichloromethane. The extracts were concentrated and applied to a thin-layer chromatography (TLC) plate (60F254; Merck, Darmstadt, Germany) with non-radioactive standard steroids as carrier steroids, and developed in a mixture of benzene: acetone (4:1) and benzene: ethyl acetate (4:1). Radioactive steroid metabolites were analyzed using a BAS 1500 bio-imaging analyzer (Fuji Film, Tokyo, Japan), and estrone (E1) and E2 standards were visualized by exposure to iodine vapor. Other standard steroids were detected by UV absorption at 254 nm.
The migration zones corresponding to the carrier steroids were eluted twice from the silica plate bands with 5 ml of dichloromethane: methanol (90:10). Following centrifugation at 1,000×g for 10 min, the supernatants were vacuum dried before finally being dissolved in 20 μl of acetonitrile. Extracts were then analyzed by reverse-phase high-performance liquid chromatography (HPLC). Briefly, a Waters Alliance HPLC system (Waters, Milford, MA, USA) equipped with a binary pump (515 HPLC pump, Waters) and UV detector (2487 multi-wavelength absorbance detector, Waters) was used. Chromatographic separation was performed on a Sunfire C18 analytical column (4.6×150-mm I.D., 3.5-μm particle size; Waters Sunfire). The flow rate and mobile phase were 0.6 ml/min with 20% methanol and 0.4 ml/min with absolute acetonitrile.
The steroid metabolites were identified by gas chromatograpumass spectrometry (GC-MS). Steroids were analyzed on a DB-5MS column (60 m×0.25 mm O.D.×0.25 μm I.D.) using a Shimadzu GCMS-QP2010 mass spectrometer interfaced to GC-2010 gas chromatograph. The following conditions were used: splitless injection at 80°C (injector temperature of 280°C), followed by a 1-min delay before maximal temperature 20°C/min to program starting temperature of 250°C. Thereafter, the temperature was programmed at 5°C/min to maximum 295°C.
RESULTS
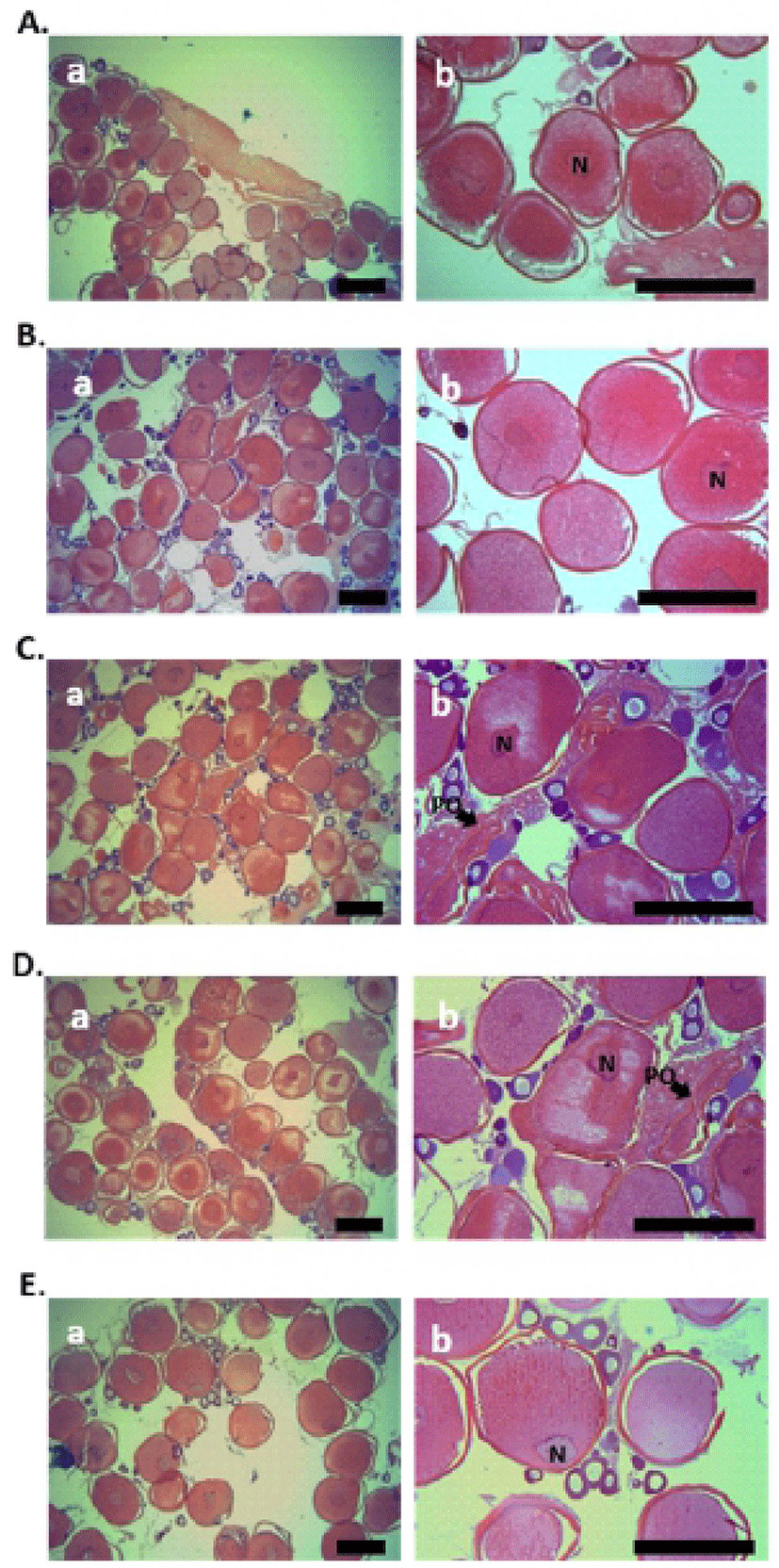
Histological analysis of fish ovary was performed using the samples, collected at five each sampling during the breeding season (January to February). In both the 0.52 and 0.55 mm diameter oocytes of starry flounder, yolk granules were spread throughout the ooplasm, and nucleus (N) was located near the center of oocytes (Fig. 1A and B). In 0.63 and 0.66 mm diameter oocytes of starry flounder, migration of N was observed (Fig. 1C and D). Later, nucleus was gradually displaced toward the animal pole in 0.71 mm diameter oocytes of starry flounder; yolk granules were increased and combined together (Fig. 1E).
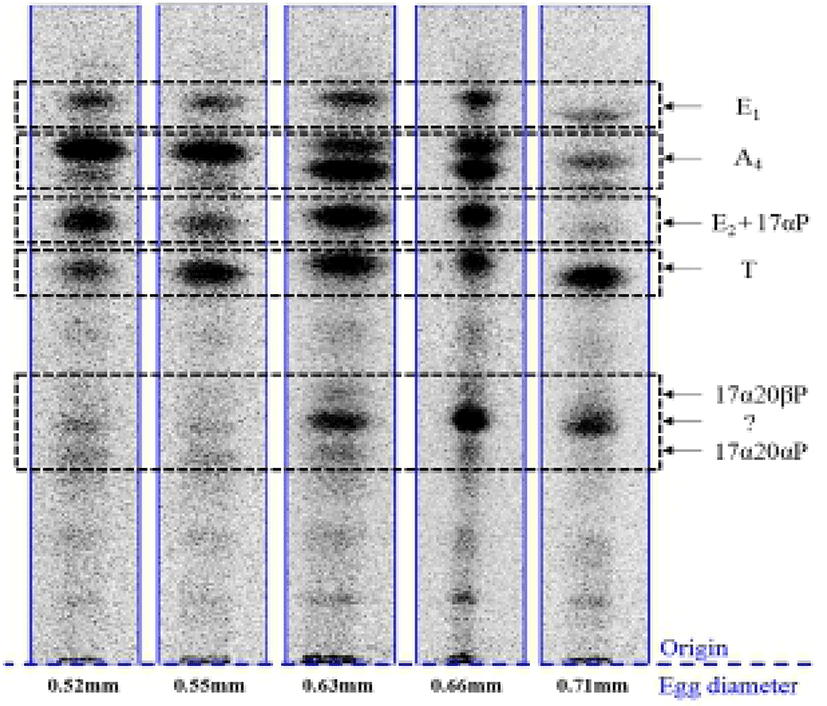
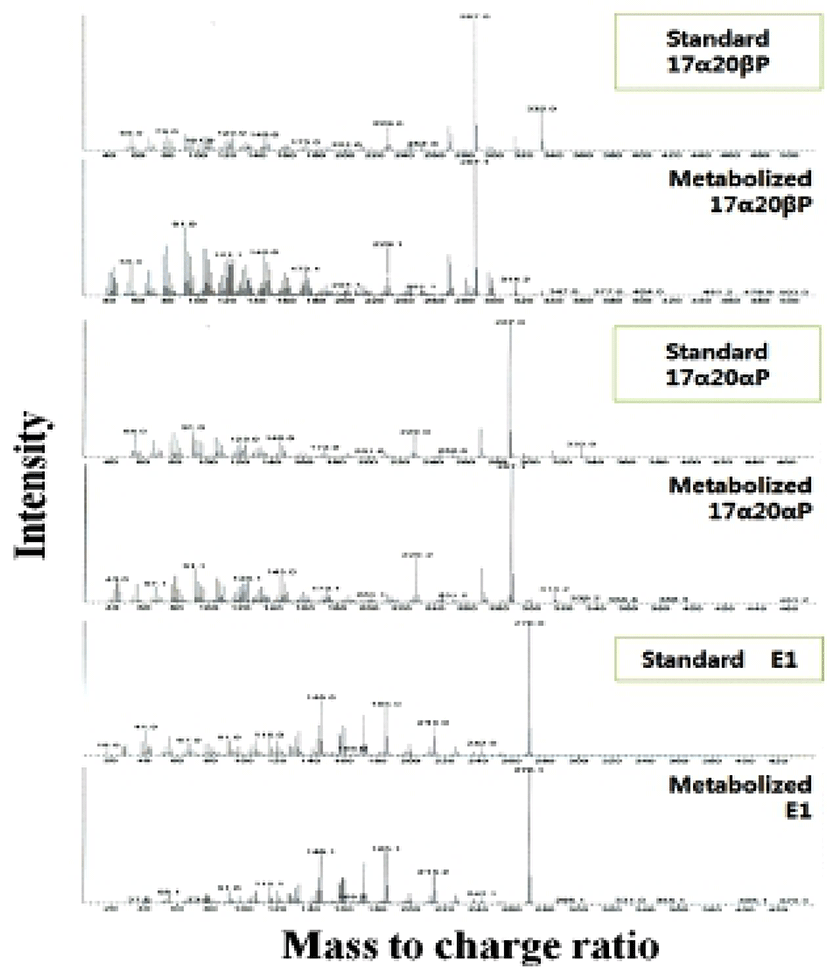
When vitellogenic oocytes (0.52 and 0.55 mm in diameter) were incubated with [3H]-17αP, the four major separated metabolites were A4, T, E1 and E2 (Fig. 2); E2 and 17αP were overlapped slightly by TLC, and E2 was further identified by HPLC. In 0.63, 0.66 and 0.71 mm diameter oocytes of starry flounder, undergoing germinal vesicle migration, unknown metabolites (?, predicted to progestin) between 17α20βP and 17α20αP were also detected. Moreover, the presence of a small quantity of estrogens was observed in the oocytes of 0.71 mm. The five zones of metabolites (converted from [3H]-17αP using the oocytes of 0.52-0.71 mm) corresponding to the carrier steroid in TLC were eluted and identified by HPLC (data not shown). From the oocytes of 0.63 and 0.66 mm it looks like there were two separated spots of A4, but it turned out be only the presence of A4. The GC-MS analysis was performed for the identification of 17α20βP, 17α 20αP and E1 (Fig. 3). The GC-MS analysis of unknown metabolites (?) in the oocytes of 0.63, 0.66 and 0.71 mm revealed the presence of 17α20βP and 17α20αP.
DISCUSSIONS
Steroid hormones play important roles in the reproduction of vertebrates. In fish, steroid hormones are critical to maintain hypothalamus-pituitary-gonad axis function, and feedback controls on the system are achieved largely through alterations in steroid production (Ankley et al., 2009). In female fish, estradiol-17β (E2), testosterone (T) and progestins are the main gonadal steroids. E2 is the major sex steroid during vitellogenesis, and progestin such as 17α20βP and 17α20β21P is important during oocyte final maturation leading to GVBD (Patiño & Sullivan, 2002; Baek, 2008; Nagahama & Yamashita, 2008).
In the present study, we verified the steroid metabolites from vitellogenic (0.52 and 0.55 mm in diameter) and mature oocytes (0.63, 0.66 and 0.71 mm oocyte diameter) with [3H]-17αP as a precursor. The major metabolites produced from the oocytes of starry flounder were A4, T, E2, E1, 17α20βP and 17α20αP. From these steroids, we were interested in E1 and 17α20αP.
In the most of species, the main estrogen is E2, but vitellogenic oocytes of starry flounder also synthesized E1 as a major steroid metabolite. This metabolite was produced high through the entire period of maturation. From just prior to GVBD (in the oocytes of 0.71 mm), trace amounts of estrogens was detected. E1 has about one fifth activity of E2 in inducing trout vitellogenin synthesis (Routledge et al., 1998). Similarly, Specker & Sullivan (1994) reported that E1 may have a minor vitellogenic role in some species. In red seabream, in vitro conversion of E1 into E2 was 16-fold greater than T conversion into E2, suggesting that E2 is synthesized mainly via E1 rather than through T (Ohta et al., 2002). The steroidogenic pathways for estrogen production are different among species. Further studies should be performed physiological roles for intermediate products other than T in the E2 synthetic pathway.
When starry flounder oocytes (0.63, 0.66 and 0.71 mm oocyte diameter) were incubated with [3H]-17αP, two additional metabolites were appeared, 17α20βP and 17 α20αP. The activity of 17α20αP, an isomer of 17α 20βP, was higher than 17α20βP in the migratory oocytes (data not shown). But 17α20β21P was not detected. In this species, two progestins, 17α20βP and 17α20αP, likely act as MIS.
A large amount of 17α20αP has been found in the blood and ovarian incubates of mature flatfish species (Canario & Scott, 1990; Scott & Canario, 1990), and cyprinids (Kime et al., 1992). These authors suggested that it is not a potent inducer of oocyte maturation. The role of this steroid is still unknown.
Until now, there are two major MIS identified in fish, 17α20βP and 17α20β21P. They both probably act as MIS in most fishes, but normally one of them is the predominant MIS for a given species. The 17α20βP is the major MIS in several salmonid and non salmonid species, while 17α20β21P is the major MIS in Atlantic croaker, spotted sea trout, striped bass and black porgy (Yueh et al., 2005).
Data presented in this study suggest that metabolite 17 α20αP may have some roles during oocyte maturation process.