INTRODUCTION
We adopted the experimental marine medaka, Oryzias dancena. Because the marine medaka is gaining attention as an experimental animal in the aquaculture. This fish is a truly euryhaline teleost, with a great capacity for hypo- and hyperosmoregulation. It also has a short interval between generations, with spawning possibilities just 60 days after hatching. Most of its physiological attributes are similar across a wide spectrum of salinities, ranging from complete freshwater to normal seawater (Inoue & Takei, 2003; Kang et al., 2008; Cho et al., 2010). Much attention has been directed toward extending the utility of functional transgenic marine medaka strains for ornamental purposes because they can be used at most naturally occurring salinities (Cho et al., 2011).
The induction of triploidy has been achieved in a number of different freshwater and marine fish species (Felip et al., 2001). The main benefit of triploidy is sterility condition. Sterility allows organisms to avoid the metabolic costs of sexual maturation, resulting in continued somatic growths of triploid fish, with maintenance of flesh quality during the period when the diploids sexually mature. In addition, sterility prevents fish mortality related to spawning (Benfey, 1999). Because of these advantages, the induction and rearing of triploid fish have been practiced in the aquaculture of several economically relevant species (Hulata, 2001). Furthermore, sterile triploid fish are unable to breed and contribute to the local gene pool if they escape from the confinement. By conferring in the desired introduction of exotic fish species for limited purposes, triploidy can serve as an effective method by which to reduce or eliminate the environmental risks of genetically modified organisms (Dunham & Devlin, 1999).
Triploid has many advantages. Induced triploid by blocking the second meiosis has been proposed as one approach for the generation of transgenic fish with depressed reproductive capacities (Piferrer et al., 2009). Induced triploid fish have impaired gametogenesis and investments in somatic growth may not be hindered by the metabolic costs of sexual maturation. Additionally, sterility of induced triploid may be the means to prevent the decline in flesh quality associated with sexual maturation, and it also addresses concerns regarding the environmental impact of farmed escapees (Peruzzi et al., 2004).
An important consequence of increased nuclear and/or cellular volume in triploid fish is the decreased ratio of surface area to volume. This could affect processes limited by the surface area, such as nutrient and metabolite exchanges, passive and active ion exchanges, and membrane binding of hormones and other messengers. Due to decreased cell numbers, this decreased ratio of surface to volume also applies to whole tissues and organs (Benfey, 1999). A second important consequence of increased nuclear and/or cellular volume is that, depending on the shape of the cell and its nucleus, the internal transport and diffusion distance may be increased. This could affect processes such as signal transduction from the cell surface to the nucleus, as well as the resultant production and movement of RNA and proteins within and outside the nucleus and cell (Benfey, 1999). Some of these potential disadvantages of triploid cells may be offset by energetic advantages arising from reduced production and maintenance of cellular membranes and from the smaller relative surface area across where the ionic and osmotic gradients must be maintained (Benfey, 1999).
Futhermore, there are numerous studies in the literature which have investigated various aspects of induced triploid fish identification methodology including analysis of the measurement of erythrocyte and nuclear size, the distinction of nucleolar number, the measurement of cell number, and the measurement of cell and nuclear size in different tissues (Benfey, 1999; Park & Kim, 2000). For this reason, the purpose of this study is to determine and to compare whether diploid and induced triploid marine medaka are different in terms of main hematological and histological characteristics.
MATERIALS AND METHODS
Experimental group of diploid marine medaka, Oryzias dancena in this study were reared by methods of Park et al. (2011). On 24 September 2013, the one hundred fishes were quarantined by the male and female categories and habituated in the 100 L glass aquariums for 3 days. The sex ratio of males and females was 60 males and 40 females. For collecting eggs, the fish whose standard length were over 25 mm used in this experiment and 35 males and 15 females of marine medaka were placed in each of two aquariums, and 1,000 fertilized eggs were collected immediately by net. The fertilized eggs of diploid experi-mental group (n=500) were reared in 100 L glass aquarium. Induced triploid was induced in the marine medaka by the cold shock treatment (4°C) of fertilized eggs for two minutes after fertilization of 45 minutes (Ko, 2013). The induced triploid genotype was induced by all thermal shock regimes tested. Induced triploid was confirmed with chromosomal and erythrocyte measurements and also the flow-cytometric analyses by using flow cytometry (PA-II, Partec, Germany).
We extracted the eye, kidney and midgut epithelium needed for histological observations from each species. Each sample was fixed in Bouin's solution during the day and washed with flowing water. For decalcification, they were processed in decalcification solution for 24 hrs, and then, washed again. Next, in the order of 70% alcohol, 80% alcohol, 90% alcohol and 100% alcohol used for dehydration of 1 hour each. Clearing the xylene for impregnation , they were treated with both soft paraffin and hard paraffin. After impregnation, samples were embedded, trimmed and cut. At this time, each barbell of the upper, central and lower parts were by a 6 μm thickness across and longitudinally. Afterwards, they were stained with hematoxylineosin staining. Next, the samples were mounted with Canadian balsam and all processes were completed. We took pictures with an optical microscope camera (Axiocam MR, Carl Zeiss, Germany) after scrutinizing by optical microscopy (Axiostar Plus, Carl Zeiss, Germany). Using an eyepiece micrometer under an optical microscope, we used the methods of Park et al. (2006) to measure the thickness of the retina; the epithelial layer (EL), the rod and cone layer (RCL), the outer limiting membrane layer (OLM), the inner nuclear layer (INL), the inner plexiform layer (IPL), and the ganglion cell layer (GCL). To analyze the development of the kidney and midgut epithelium, we used the Axioskop 4.1 image analysis software (Carl Zeiss, Germany) to measure the areas and volumes of the cells and nuclei with the following formulae: surface area = 1/4 × abπ, and volume = 4/3 × π(a/2) × (b/2)2, where a is the major axis of the cell or nucleus; and b is the minor axis of the cell or nucleus (Park & Kim, 2000).
The experiment was performed in triplicate and the results are reported as means of ±SD (n=30), unless otherwise stated. The data were analyzed by one-way ANOVA when using the SPSS statistical package (SPSS 9.0, SPSS Inc., Chicago, IL, USA). Means were compared by using Duncan's multiple range test, and were considered to be significantly different at P < 0.05.
RESULTS
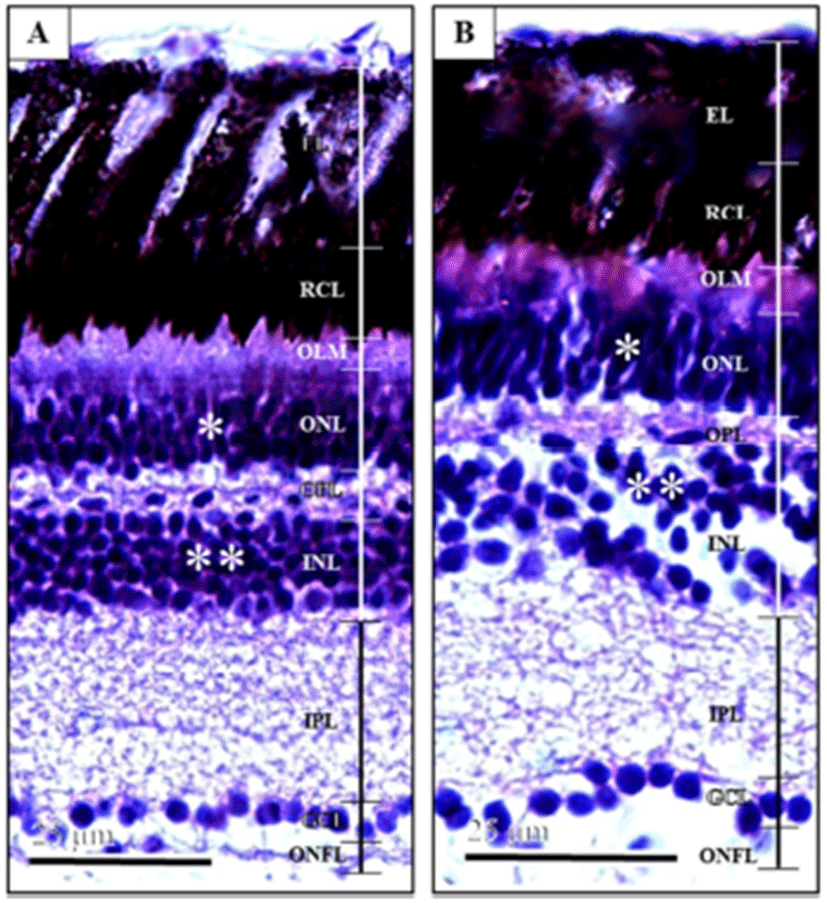
Fig. 1 showed the retina layer of marine medaka, Oryzias dancena eyes. The thickness of retina was about 1.31 times different. The ratio of the length was different for each layer, but triploid was measured to be longer than diploid from all layers. Outer limiting membrane (OLM) consists of cone photo-receptors with a shorter tapered outer segment, larger ovoid ellipsoids containing an oil droplet, and nuclei. Outer segments of photoreceptors were partially enveloped by projections of the pigment epithelium. It has a network-like structure and is situated at the base of the rods and cones. Outer nuclear layer (ONL) consists of the nerve fibers leading to the optic tract. There was also a difference in the cellular structure between triploids and diploids. In triploids, the outer nuclear layer consisted of two strata of nuclei, while in diploids, the same layer consisted of three strata of nuclei. Outer plexiform layer (OPL) is organized as a thin reticular tissue. Inner nuclear layer (INL) has horizontal, amacrine, and bipolar cells. Inner plexiform layer (IPL) consists of reticular tissues. Ganglion cell layer (GCL) contains the perikarya of ganglion cells and dis-placed amacrine cells. Optic nerve fiber layer (ONFL) consists of the nerve fibers leading to the optic tract (Park et al., 2006). Retina forned layer of cells along the inside lining of the eye. Induced triploid thickness of retina are smaller than diploids, and induced triploid ratio of retina OLM was 1.22 times, ONL was 0.98 times, OPL was 1.07 times, INL was 1.22 times, IPL was 1.01 times, GCL was 1.19 times, ONFL was 1.12 times larger than diploid measured trends (Table 1).
| Diploid | Induced triploid | Ratios of means | |
|---|---|---|---|
| Thickness of retina (µm) | 110.9±2.15a | 97.7±2.50b | 0.88 |
| Thickness of each layer of retina (%) | |||
| Epithelial layer | 22.5±0.89a | 22.6±1.52a | 1.01 |
| Rod and cone layer | 11.9±0.85a | 12.9±0.95b | 1.08 |
| Outer limiting membrane | 4.5±0.40a | 5.5±0.32b | 1.22 |
| Outer nuclear layer | 12.3±0.43a | 12.0±0.98a | 0.98 |
| Outer plexiform layer | 4.1±0.21a | 4.4±0.58b | 1.07 |
| Inner nuclear layer | 13.1±0.44a | 16.0±0.21b | 1.22 |
| Inner plexiform layer | 23.0±0.42a | 23.2±0.22a | 1.01 |
| Ganglion cell layer | 4.9±0.14a | 5.8±0.46b | 1.19 |
| Optic nerve fiber layer | 5.7±0.56a | 6.4±1.03b | 1.12 |
| Number of outer layer cellnucleus | 3a | 2b | |
Horizontal cell nucleus in the inner nuclear layer of retina major axis, minor axis, surface area and volume were each 1.10, 1.21, 1.33 and 1.60 times larger than diploid, and appeared to be significantly different (Table 2) (P<0.05). Also, ganglion cell nucleus in the ganglion cell layer of retina major axis, minor axis, surface area and volume were each larger than 1.29, 1.24, 1.60 and 2.00 times of diploid, and appeared to be significantly different (Table 2) (P<0.05). On the other hand, induced triploid horizontal cell nucleus and ganglion cell nucleus show lower density than diploid (Fig. 1).
| Diploid | Induced triploid | Ratios of means | |
|---|---|---|---|
| Horizontal cell nucleus in inner nuclear layer ofretina** | |||
| Major axis(µm) | 3.7±0.28a | 4.0±0.33b | 1.10 |
| Minor axis(µm) | 2.2±0.11a | 2.6±0.13b | 1.21 |
| Surface area(µm2) | 6.3±0.69a | 8.3±0.87b | 1.33 |
| Volume(µm3) | 9.1±0.79a | 14.6±1.44b | 1.60 |
| Ganglion cell nucleus in ganglion cell layer ofretina** | |||
| Major axis(µm) | 3.2±0.14a | 4.1±0.46b | 1.29 |
| Minor axis(µm) | 2.9±0.26a | 3.6±0.23b | 1.24 |
| Surface area(µm2) | 7.2±0.90a | 11.5±1.10b | 1.60 |
| Volume(µm3) | 14.0±1.29a | 27.9±1.78b | 2.00 |
Each values are the means standard deviation of triplicated groups. Means in rows with the different superscript letter are significantly different (P < 0.05), Ratios of means = induced triploid/diploid.
Surface area= 1/4 × abπ and volume = 4/3 × π(a/2) × (b/2)2 (where a = the major axis of a cell or nucleus; b = the minor axis of a cell or nucleus; after Park and Kim, 2000).
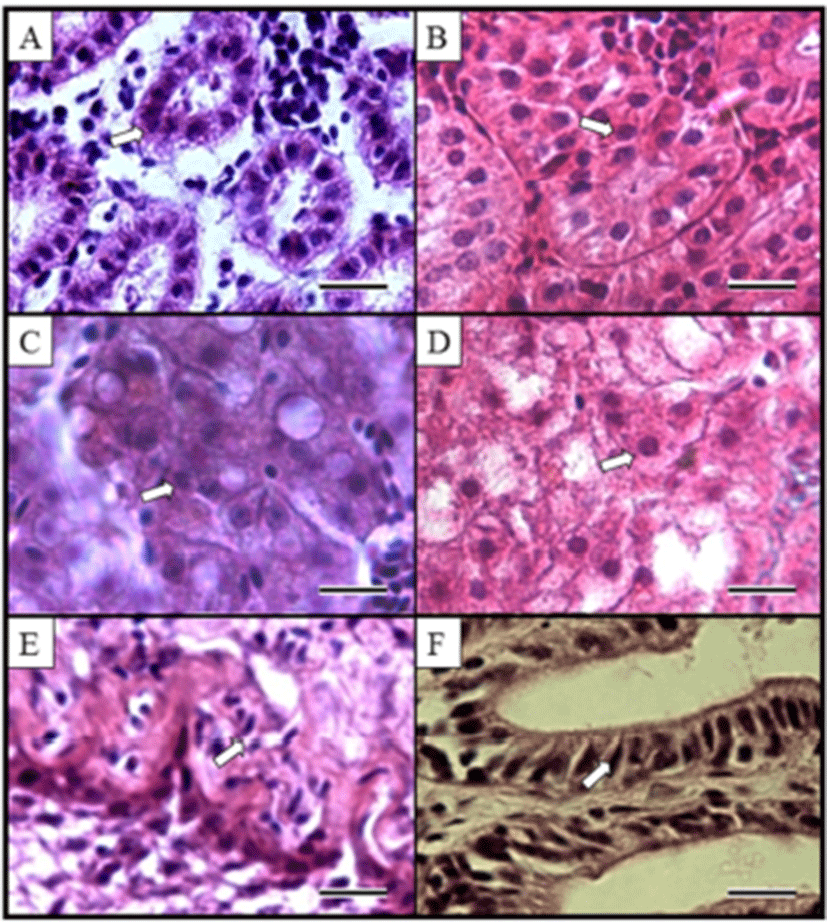
Kidney, liver and intestine of diploid and induced triploid were compared at Fig. 2. We observed histological sections for proximal tubule cell of kidney, hepatocytes of liver and midgut epithelium. From observed results, the induced triploid was larger than diploid. The histological structure of marine medaka liver and intestine major axis ratios of diploid and induced triploid of kidney were 1.19 times, and the surface area was 1.74 times where the major axis and minor axis ratios of hepatocytes were 1.29 times and 1.52 times. Ratios on nuclear height of midgut epithelium appeared to be 1.20 times (Table 3). Each of induced triploid cell and nucleus size are shown to be bigger than diploid, but the induced triploid cell and nucleus appeared as low density (P<0.05).
| Diploid | Induced triploid | Ratios of means | |
|---|---|---|---|
| Proximal tubule cell ofkidney** | |||
| Major axis(µm) | 3.7±0.28a | 4.7±0.33b | 1.19 |
| Minor axis(µm) | 2.1±0.11a | 2.8±0.13b | 1.19 |
| Surface area(µm2) | 6.1±0.69a | 10.7±0.87b | 1.74 |
| Volume(µm3) | 9.8±0.79a | 21.4±1.44b | 1.26 |
| Cell number in proximaltubule | 12.5±0.81a | 8.9±0.52b | 0.71 |
| Hepatocytes ofliver** | |||
| Major axis(µm) | 4.6±0.14a | 5.1±0.46b | 1.29 |
| Minor axis(µm) | 3.4±0.26a | 4.1±0.23b | 1.52 |
| Surface area(µm2) | 16.6±1.90a | 16.7±3.40b | 1.00 |
| Volume(µm3) | 47.1±8.29a | 46.3±5.18b | |
| Nuclear height of midgut epithelium | 4.8±0.25a | 5.8±0.21b | 1.20 |
Each values are the means standard deviation of triplicated groups. Means in rows with the different superscript letter are significantly different (P < 0.05), Ratios of means = induced triploid/diploid.
Surface area= 1/4 × abπ and volume = 4/3 × π(a/2) × (b/2)2 (where a = the major axis of a cell or nucleus; b = the minor axis of a cell or nucleus; after Park and Kim, 2000).
DISCUSSION
We took blood and tissues (kidney, liver, intestine and retina) samples of marine medaka, Oryzias dancena, and then each of diploid and induced triploid cells were compared. Method of induced triploid fish was well known with chemical treatment such as colchicine and cytochalasin B, or temperature treatment to fertilization of eggs or physical treatments that were applied with pressure. Chemical treatments are often used in plants and shellfish, physical treatments are used in fishes (Thorgaard, 1986). We have experimented with physical treatments in this study.
Although the triploid fish appeared to be 1.5 times with chromosome increased and bigger cell size phenomenon than diploid, but the body size did not present gigantism. This phenomenon reported by Swarup (1959b), the triploid stickleback, Gasterosteus aculeatus, cartilage, blood and neuron cell and nuclear size increased as compared to diploid, but these results not affect body size gigantism. Triploid has red blood cells and nuclear sizes bigger than diploids while the triploid number of red blood cells decreased more than diploid, this reason offsets the body size gigantism effect, and triploid fish which causes red blood cell enzyme activity reductions, and lowers oxygen transport (Ueno, 1984; Sezaki et al., 1988; Park & Park, 1995).
The number of outer nuclear layer was three in diploid, while two appeared in triploid. These result observed other species, stickleback, Gasterosteus aculeatus and sweet fish, Plecoglossus altivelis (Swarup, 1959a; Aliah et al., 1990). Triploid thickness of outer nuclear layer percentage was smaller than diploid, and also retina thickness was small as compared to diploid. In induced triploid, the rod and cone layer percentage for thickness of retina was 1.08 times bigger than diploid, and similar results which appeared in triploid sweet fish increased when compared to diploid sweet fish (Aliah et al., 1990). In case of diploid, the sweet fish nuclear number of rod and cone layer was much more than triploid sweet fish. For this reason, the induced triploid sweet fish indicated low accuracy of visual as compared to diploid (Aliah et al., 1990). Also, the adverse aspects of induced triploid showed rainbow trout, Oncorhynchus mykiss, where induced triploid rainbow trout easily feels stress. During mix breeding, the induced triploid rainbow trout is turned over for feeding competition as compared to diploid (Aliah et al., 1990; Lincoln & Bye, 1984). Triploid horizontal cell nucleus found in the inner nuclear layer of retina major axis, minor axis, surface area and volume and ganglion cell nucleus in ganglion cell layer of retina major axis, minor axis, surface area and volume all increased due to chromosome haploid increases (Thorgaard, 1986; Aliah et al., 1990).
We measured proximal tubule cells of the marine medaka kidney. Major axis, minor axis and volume have small differences for each sample lengths. The surface areas of induced triploid samples are 1.74 times longer than diploid samples. This is the longest ratio of differentiation with induced triploid and diploid. Measured hepatocytes, the largest ratio value of diploid and induced triploid are minor axis, which is 1.52 times. The least ratio value of surface area is 1.00 times.
Overall, induced triploid fish is bigger than diploid fish and ratio values are a variety of distribution. Thus, we can find the percentage differences of diploid and induced triploid tissues. In previous studies, many organs and tissues have larger but fewer cells in induced triploids, including the brain, muscle, retina, liver and kidney (Benfey, 1999). This arises due to the extra set of chromosomes dictating an increase in cell nucleus dimensions which affects overall cell size.
As a result, this dissertation suggests that some tissues of induced triploid are larger than those of diploid in cells and muscles. This is infertility that is related to induced triploid features. Sterile induced triploid used less energy in the gonad maturity (Cal et al., 2006). Therefore, the fish meat quality is high and of good taste because of the energy used growth. Therefore, industry of induced triploidization of high quality fish such as salmon, and flounder is a common trend (Kim & Nam, 2001). From this trend, we obtained histological data of induced triploid tissues. Our results can be used in the future as the baseline data for measuring the histological comparisons of diploid and induced triploid tissues in fish.
In this study, induced triploid marine medaka showed increased sizes in red blood cell and nuclear, horizontal cell nucleus in inner nuclear layer of retina major axis, minor axis, surface area and volume and ganglion cell nucleus in ganglion cell layers of retina major axis, minor axis, surface area and volume. On the other hand, the number of outer nuclear layers in retina and nucleus number in proximal tubule of kidney decreased like other triploid fish. The increased size of cell and nucleus and the decreased number of cell and nucleus in some tissue phenomenon are useful for indicating the use of ploidy distinctions.
