INTRODUCTION
It is suggested that the black soybean can be used in the development of foods for preventing obesity (Kim et al., 2013, 2015). In addition, it is a useful oriental medicine in medical history. A black soybean seed extract suppresses fat accumulation in visceral adipose tissue, reduces the plasma glucose level, and enhances insulin sensitivity. In addition, in visceral adipose tissue, it also decreases food intake and the expression of lipogenesis genes, and increases the levels of lipolysis proteins without toxicity (Kanamoto et al., 2011; Kim et al., 2015). The components of black soybeans are not that different from yellow soybeans, but there is much more anthocyanins in black soybean than the yellow soybean (Lee et al., 2009; Cho et al., 2013). The testa of black soybeans has beneficial properties such as anti-oxidative, anti-carcinogenesis, and anti-inflammatory effects. Anthocyanins, the polyphenolic pigments are the main components of testa which is the impart color of soybean.
Although, the contents of the extracted black soybean testa depends on the type of black soybean, the Cheongia #3 type of black soybean contains seven anthocyanins, with dominant levels of cyaniding-3-O-glucoside (C3G), delphinidin-3-O-glucoside (D3G), and petunidin-3-O-glucoside (P3G) (Lee et al., 2009; Ha et al., 2010). It is suggested that the antiobesity effects of black soybeans in mammalsfed high-fat diets depends on their anthocyanins (Tsuda et al., 2004; Kwon et al., 2007; Kim et al., 2012; Sato et al., 2015). Anthocyanins enhance adipocytokine (adiponectin and leptin) secretion and up-regulate the adipocyte specific gene expression without activation of PPARg in rat adipocytes through AMP-activated protein kinase activation (Tsuda et al., 2004).
Obesity depends on the increase of adipocyte size and number (Colitti & Grasso, 2014). Because adipocyte size is usually restricted in adipose tissue which depends on species and strain, the number of adipocytes is one of the key factors to the size increase of adipose tissue (Obst et al., 1981; Ryden et al., 2014). The subcutaneous adipose tissue of mouse is constructed with white adipocyte and white adipose tissue (WAT) is a dynamic and modifiable component in body (Hausman et al., 2001). WAT is developed in late stage of human gestation (Ailhaud et al., 1992), meanwhile, it is developed at postnatal development in mice (Pouteau et al., 2008). It is well known that the growth of this tissue is limitless in mammals under states of persistent energy surplus (Parlee et al., 2014).
While the expected antiobesity effects are the suppression of hypertrophy and hyperplasia of adipocyte, the unlimited growth of WAT suggests the possibility of involvement of mesenchymal stem cells in adipose tissue (ASC) in addition of precursor cells and adipocyte. To evaluate the possibility, in this study, the characters of adipose derived stem cell (ADSC) was examined by in high-fat diet induced obese mice with or without administration of the ethanol testa-extract of Cheongja #3 black soybean (ETCBS) and in isolated ADSC.
MATERIALS AND METHODS
The ethanol extract of the testa of Cheongja #3 black soybean (ETCBS) was performed as previously described (Kim et al., 2015). C57BL/6N mice (4 weeks old) fed with 45% high-fat diet (Research Diets, New Brunswick, NJ) to induce obesity. After 10 weeks, obesity induced mice (n=30) were randomly assigned into two groups; a high-fat diet group and a high-fat diet group with 1g/kg BW of ETCBS. Vehicle (H2O) and ETCBS were exposed by oral administration for 12 weeks.
All experimental animals were housed in the standard condition and fed a standard rodent diet and water ad libitum at Sungshin University with 14 h light – 10 h dark light cycle and handled in accordance with the guideline of care and use of laboratory animals. Animal body weight and food intake were measured twice per week throughout the experiment. After 12 weeks, the mice were fasted for 15 h and sacrificed.
Mouse subcutaneous adipose tissues were isolated from each treatment group and isolated the mesenchymal stem cells as described previously (Jeon et al., 2013). In briefly, the adipose tissues were washed with Hank’s buffered salt solution (HBSS) containing 1% BSA, 200 nM adenosine, and 50 mg/mL glucose. It was minced finely using scissors and incubated in digestion buffer at 37°C for 1 h with constant agitation. After digestion the mononuclear cells were washed and seeded. mADS cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Cat # 31600-026) containing 10% fetal bovine serum (Welgene, Cat # S001-07), 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 3.7 mg/mL sodium bicarbonate. During maintaining the medium was changed every other day. Passage was performed at 90–95% confluence. The number of mADS cells was measured with hemocytometer after 0.4% trypan blue (Sigma-Aldrich, USA) staining. The dead cells were excluded in doubling time measurement. Doubling time was assessed from P1 to P4 in standard mADS cells culture medium. Data obtained from each group was reported as mean of the values and repeated three times.
Cell proliferation was monitored using [3-(4,5-dimethylthiazol- 3yl)2,5-diphenyltetrazolium] bromide cell proliferation assay kit (Cat #: 4891-025, Trevigen, Maryland, USA). Cells (1 × 104) subcultured at 96 well plates with six replicate wells. Cell proliferation was measured following the manufacturer’s protocol. 6 h after seeding, cells were treated with (50 ㎍/mL, 100 ㎍/mL, and 200 ㎍/mL) or without ETCBS. The optical density was measured on 570 nm wavelength spectrophotometer.
mADS cells were cultivated in DMEM supplemented with 10% FBS, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 3.7 mg/mL sodium bicarbonate at 5% CO2 in air, 37°C, and > 95% humidity. At 95% confluence, the medium was changed with adipocyte induction medium (DMED containing 10% FBS, 10 μM insulin, 0.5 mM isobutilmethyilxanthin, 1 μM dexamethasone, and 200 μM indomethacin) and cultured for 14 days with (50 ㎍/mL, 100 ㎍/mL, and 200 ㎍/mL) or without ETCBS.
The adipogenic phenotype was determined by staining with 2% Oil Red-O solution. The quantity of accumulated triglyceride was measured with microplate reader at 540 mm wavelength after isopropanol extraction of Oil Red-O.
RESULTS
To evaluate the different characteristics of adipose derived stem cells by the origin and the administered chemicals, the adipose tissues were cut out from subcutaneous and visceral areas. The doubling time was different between subcutaneous and visceral ADS cells. In control group, the doubling time of visceral ADS cells at P0 (62.3 h) was longer than that (45.7 h) of subcutaneous ADS cells (Fig. 1). By the in vitro culture and passage, the doubling time was increased both subcutaneous ADS cells (60.3 h) and visceral ADS cells (80.5 h) (Fig. 1). At P0 the doubling times of control, high fat diet, and ETCBS were similar (45.7 h, 51.6 h, and 43.6 h, respectively) in subcutaneous ADS cells. Administration of ETCBS did not increase the doubling time of them (Fig. 1).
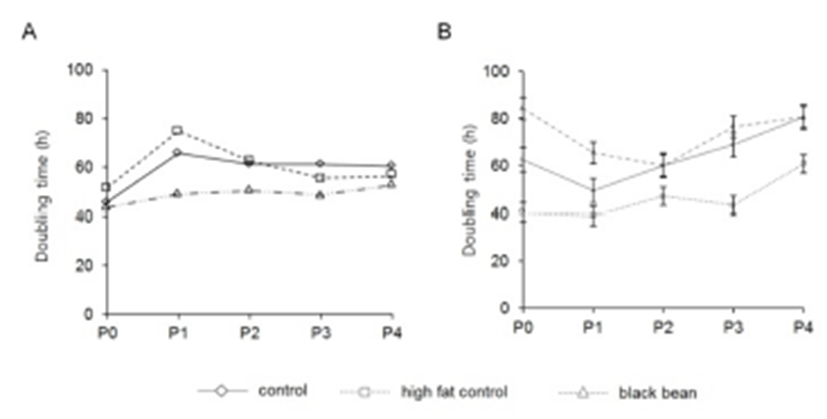
In the case of visceral ADS cells, the doubling time was 62.3 h or 40.3 h in control or high fat diet administrated mice, respectively. ETCBS administration resulted in increasing the doubling time from 62.3 h to 84.2 h (Fig. 1). At passage 4 the doubling time was become similar between control and ETCBS but not in high fat control (60.6 h) (Fig. 1). This result indicates that the origin of ADS cells influences on the proliferative activity. However, the characters of those two populations were become similar between the groups if there is no more treatment of chemicals.
To evaluate the effects on the shape by the administration of high fat-diet and ETCBS, the subcutaneous ADS cells were culture under the standard condition. The characters of shape in subcutaneous adipose derived stem cells were not affected by high fat-diet or ETCBS. The shape was not different between groups at the initial time (Fig. 2A). After passage 4, the shapes of ADS cells were not different between groups. The cells could growth in stratified shape in all groups (Fig. 2A). In the case of visceral ADS cells, their shape was more round than that of subcutaneous ADS cells. As the results of subcutaneous ADS cells, ETCBS and high fat-diet did not change the shapes of the ADS cells. The shape was maintained over passages.
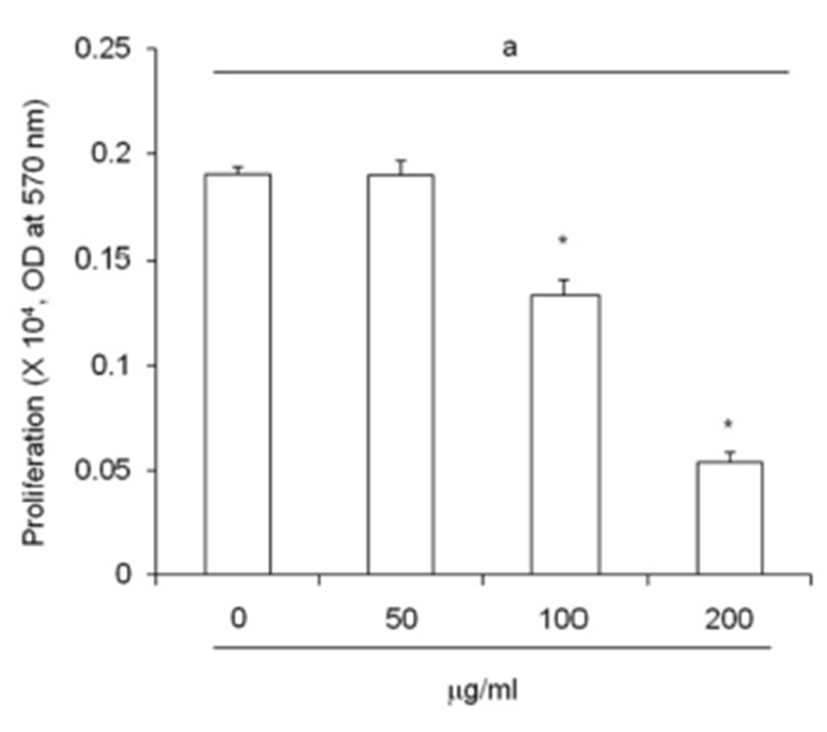
An increased of adipose precursor cell number is one of primary causes of obesity. ETCBS suppressed cell pro-liferation activity by MTT assay (Fig. 3). The activity of subcutaneous ADS cells were decreased in dose dependent manner. The activity was significantly low in 100 ㎍/mL and 200 ㎍/mL (Fig. 3).
To evaluate the meaning of the responding characters of subcutaneous ADS cell to ETCBS such as the decrease of cellular activity but the no change in doubling time, subcutaneous ADS cells were treated with ETCBS at the indicated concentrations during adipocyte differentiation for 21 days. Subcutaneous ADS cells were induced by standard adipogenic induction media with or without black bean extract. The ETCBS had effect on the adipogenesis of subcutaneous ADS cells . As seen in the photomicrograph, the intensity of Oil Red O staining was very faint in 100 ㎍/mL and 200 ㎍/mL treated groups (Fig. 4A).
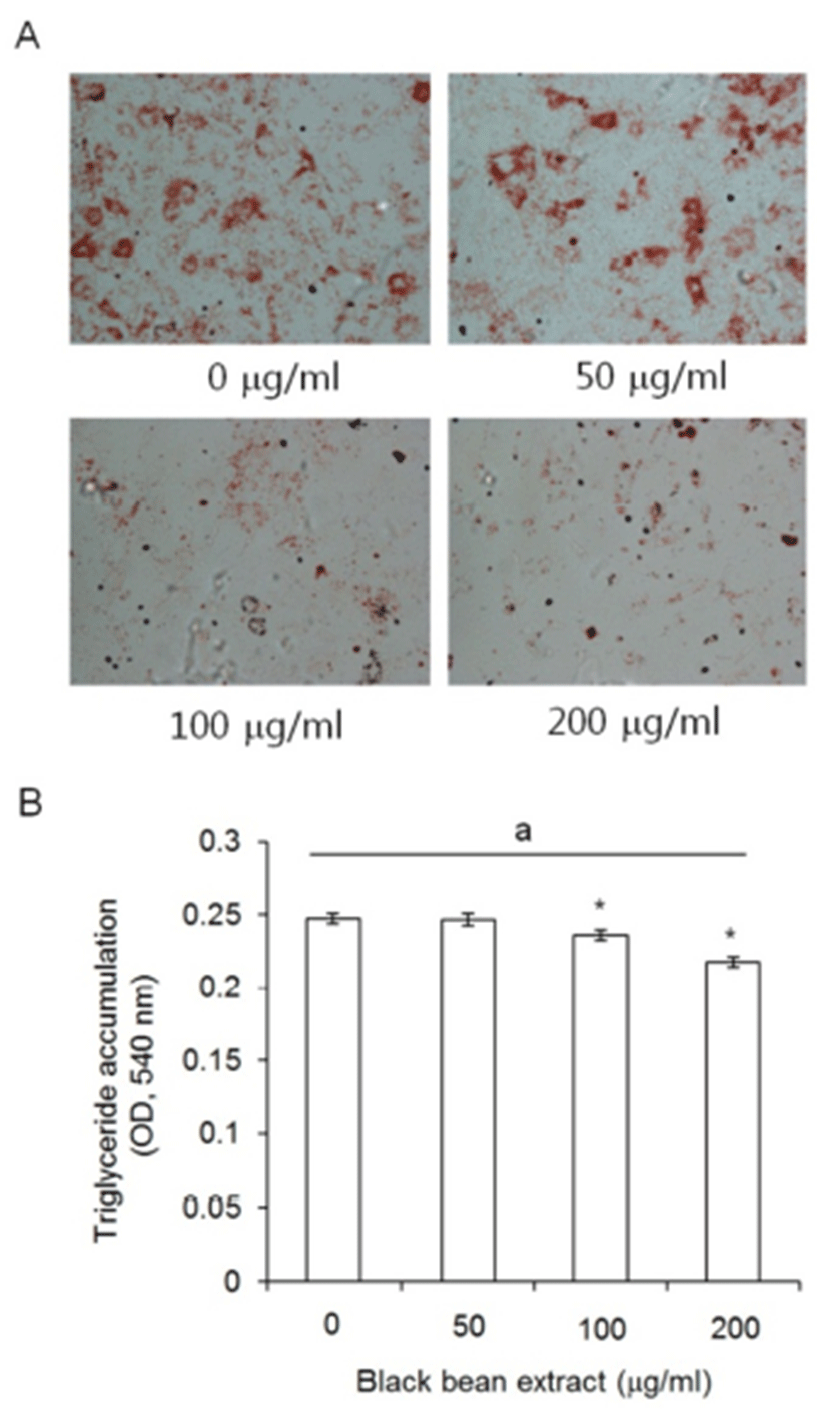
The amount of the accumulated triglyceride in the adipogenic cells was measured and significantly low in 100 and 200 ㎍/mL treated groups as expected at Fig. 4A. The OD at 540 nm were 0.243 ± 0.004 in the control and 0.246 ± 0.004 in 50 ㎍/mL treatment group with no significant differences (P = 0.938). In 100 and 200 ㎍/mL groups, the OD was 0.236 ± 0.004 or 0.217 ± 0.003, respectively, and there was statistically significant difference (P = 0.032 and P < 0.001, respectively) (Fig. 4B).
DISCUSSION
The mass of WAT is accomplished by the increase in the size and number of adipocytes. The balance of the activity of lipogenesis and lipolysis genes is the key in the regulation of the adipocyte size. Recently it has been reported that the black soybean seed testa extract upregulates the expression levels of uncoupling protein 2 (UCP-2), acetyl CoA carboxylase, and CCAAT-enhancerbinding protein alpha in visceral white adipose tissue (Kanamoto et al., 2011; Kim et al., 2015). On the other hand, A C3G-containing diet reduces body fat accumulation by activating the AMP-activated protein kinase (AMPK) in related pathways along with pro-inflammatory adipocytekines (Takikawa et al., 2010; Kanamoto et al., 2011). Besides, anthocyanine treated adipocytes have different gene expression profiles (Tsuda et al., 2005) and secretes adiponectin and leptin through AMP-activated protein kinase activation (Tsuda et al., 2004). Taken together, we suggested that the anthocianins in the testa of black soybean has similar roles in adipogenesis.
Cheongja #3 black soybean is very rich in anthocyanins (Ha et al., 2010). The ethanol testa-extract of this soybean is also the highest ratio of anthocyanins compared with those in other black soybean (Kim et al., 2012). It is known that an extract of back soybean suppresses the proliferation of human AGS gastric cancer cells (Zou & Chang, 2011). The anthocyanins of black soybean have inhibitory effects on the proliferation of both preadipocytes and maturing postconfluent adipocytes (Kim et al., 2012). Interestingly, in the case of ADS cells, the effects of the extract on the doubling time were different by their anatomical origins. The visceral ADS cells were slowly proliferated than the subcutaneous ADS cells. The extract of back soybean ETCBS induced decreasing the doubling time in visceral ADS cells but in subcutaneous ADS cells. The difference of doubling time and the response to ETCBS may reflect the different characters of ADS cell by their anatomical origins. Lipolytic activity, fatty acid metabolic characteristics, and gene expression profiles are different by the anatomical origins (Peptan et al., 2006). Taken together, it is suggested that the effects of back soybean on obesity depend on the anatomical sites.
On the other hand, it has been suggested that various dietary have bioactivity such as phytochemicals targets different stages of adipocyte life cycle, preadipocytes, maturing preadipocytes and mature adipocytes (Rayalam et al., 2008). The ETCBS suppressed the activity of subcutaneous ADS cells with a concentration dependent manner. Simply this result may suggest the suppressing effects on the proliferation of subcutaneous ADS cells. However, as seen in the doubling time of this ADS cells we clearly know that this simple suggestion is not reliable. Kim et al. (2015) previously showed that the ethanol extract of back soybean suppressed the accumulation of triglyceride. Based on this study, we analyzed the adipogenic ability of the subcutaneous ADS cells with and without ETCBS. Interestingly, most of the subcutaneous ADS cells did not undergo adipogenesis by the treatment of ETCBS. The amount of accumulated triglyceride was dramatically decreased in the ETCBS treated cells. Our results are consistent with the previous report that black soybean seed coat extract suppresses fat accumulation in mesenteric adipose tissue in the high-fat diet-fed mice (Kanamoto et al., 2011).
Although further studies are needed to find the pathway which is involved in the suppression of stem cell activity, we found that the ethanol extract of black soybean testa suppressed the activity. However, the doubling time of subcutaneous ADS cells was not different from that of the control or high fat-diet groups. It means that ETCBS may suppress the differentiation of those stem cells into the precursor and maturing of adipocyte.
In summary, the ETCBS had effect on the ADS cells depending on the anatomical origins. The doubling time of subcutaneous ADS cells was not delayed by ETCBS administration but in visceral ADS cells. The shapes of ADS cells cultured in the plate were not similar between subcutaneous and visceral ADS cells but they kept their own general shapes during passage. The cellular activity and the accumulation of triglyceride in subcutaneous ADS cells were inhibited by the treatment of ETCBS. Based on these facts, we can know that the doubling time and the effects of ETCBS are different by the anatomical origin of ADS cells. It also suggests that the components of ETCBS such as anthocyanins may suppress the differentiation of subcutaneous ADS cells into the precursor cell of adipocyte.
