INTRODUCTION
Recurrent miscarriage or spontaneous abortion refers to the loss of pregnancy before 20 weeks of pregnancy without surgical procedure for a human. It is known to occur for many reasons including genetic, uterine, or hormonal abnormalities, reproductive tract infections, and tissue rejection, but not all of which can be identified. Some researchers reported that autoimmune disorders or inflammation is one of the possible causes of recurrent miscarriage (Ford & Schust, 2009). The immune system has vital functions during establishment and maintenance of pregnancy involving a series of complex process that require well-organized interactions between the maternal uterus and the conceptus. Several mechanisms may be responsible for protection of the conceptus from immune rejection by the maternal immune system and maintenance of immune tolerance during pregnancy (Kim et al., 2011; Zenclussen et al., 2007; Trowsdale et al., 2006; Tafuri et al., 1995). Tolerance to the genetically incompatible fetus by the maternal immune system is believed to depend on the interactions of many cytokines (Zenclussen et al., 2007), above all provoking apoptosis of activated maternal lymphocytes (Makriagiannis et al., 2001). Incomplete tolerance might therefore result in disturbed pregnancy such as spontaneous abortion and pre-eclampsia. During pregnancy, changes of cytokines produced by T cells might play an important role in maternal-fetal immunoregulation and immunostimulation (Lin et al., 1993; Aluvihare et al., 2004; Raghupathy, 1997; Piccinni et al., 1998).
Th17 cells, a new subset of helper T cells, produce the hallmark cytokine IL-17A, and play a critical role in the induction of inflammation and the pathogenesis of autoimmune diseases and organ allogeneic rejection (Crome et al., 2010; Nakashima et al., 2010b). Whereas CD4+CD25+ regulatory T cells are a unique subpopulation of T cells known to play a major role in preventing autoimmunity and toleration allogeneic organ graft (Waldmann et al., 2004). The functions of effector T cells, including Th1 cells, Th2 cells and Th17 cells, are regulated by regulatory T cells, which play a fundamental role in the establishment and maintenance of tolerance (von Boehmer, 2005). An inverse relationship between Th17 cells and regulatory T cells, and that decreased levels of regulatory T cells with increased prevalence of Th17 cells and the deregulation of Th17 cells by regulatory T cells are associated with unexplained recurrent miscarriage (Wang et al., 2010a, b). However, pregnancy and abortion process involves a complex mechanism with various immune cells as well as T cells and several neuropeptide hormones associated with pregnancy, such as leptin, ghrelin and nesfatin-1 (Gonzalez et al., 2000; Gualillo et al., 2002). The neuropeptide substances rather than immune cells are involved in the formation of early embryo implantation and fertility in the uterus through the autocrine or paracrine (Luque et al., 2014; Yoon et al., 2005).
Nesfatin-1 protein is originally known as a regulator of appetite and energy metabolism, which is expressed in hypothalamus such as the paraventricular nucleus of the hypothalamus (PVN), supraoptic nucleus (SO), arcuate nucleus (ARC), lateral hypothalamic area (LHA), zona incerta and the nucleus of the solitary tract in rats (Oh-I et al., 2006; Shimizu et al., 2009; Stengel et al., 2009a,b; Stengel et al., 2010; Yosten et al., 2012). Recently, nesfatin-1/NUCB2 was expressed not only in the hypothalamus, but also in the peripheral organ such as digestive organs (Stengel et al., 2009a,b; Goebel et al., 2009; Xia et al., 2012), adipose tissues (Ramanjaneya et al., 2010), and cardiac organ (Mimee et al., 2012). Several recent studies showed that expression of nesfatin-1 in the human, rodent and fishes reproductive system (García-Galiano et al., 2010; García-Galiano et al., 2012; Gonzalez et al., 2012; Kim et al., 2011). In the male human and rodent reproductive organs, nesfatin-1 was localized within the interstitial cells, including the Leydig cells of testis (García-Galiano et al., 2010; García-Galiano et al., 2012). Nesfatin-1 increased human choriogonadotropinstimulated testosterone secretion by rat testicular explants ex vivo (García-Galiano et al., 2012). Nesfatin-1 and its binding sites are expressed in the female mouse reproductive organs (Kim et al., 2010; Kim et al., 2011). Recently, it has been reported that nesfatin-1 expression levels are decreased significantly during pregnancy progresses in rat, suggesting that nesfatin-1 may play an important role during pregnancy and fetal development (Garces et al., 2014).
However, it is not yet clear whether nesfatin-1/NUCB2 expressed in the implantation sites is associated with spontaneous abortion and if Th17 cells present in the abortion sites can be regulated by nesfatin-1. Therefore, the purpose of this study was to examine the possible roles of Th17 cells present in the implantation sites and nesfatin-1 expressed in the uterus on spontaneous abortion using the CBA/j × DBA/2 mouse model.
MATERIALS AND METHODS
CBA⁄j female mice, BALB⁄c mice, and DBA⁄2 male mice were obtained from Koatech (Korea) 7 to 8 weeks of age and the mice were kept in cages. All cages were under controlled illumination (12:12 h light/dark cycle, lights on/ off: 6 h/18 h) and temperature (22 ± 2°C). Animals were fed a standard rodent diet and tap water ad libitum. When CBA⁄j female mice had reached 10 weeks of age, they were mated with DBA⁄2 and BALB/c males, and the morning of sighting a vaginal plug was denoted as day 0.5 of pregnancy. The CBA/j × DBA/2 mouse model of recurrent miscarriage and the CBA/j × BALB/c mice as a normal pregnancy mice control. Pregnant female mice were sacrificed on day 5.5, 10.5, 14.5 and 19.5 of pregnancy and uterine tissues surrounding the embryo were collected. Mice were euthanized by CO2 anesthesia followed by cervical dislocation. Tissues were quickly removed, and then stored at –70°C for extracting total RNA preparation or protein extraction and fixed for making the paraffin section. Animal care and experimental procedures were approved by the Institutional Animal care and the use committee at the Seoul Women’s University in accordance with guidelines established by the Korea Food and Drug Administration.
To determine the mouse estrous cycle, vaginal secretions were collected with a plastic pipette filled with 10 μL of normal saline (0.9% NaCl) by inserting the tip into the mouse vagina, repeatedly. After the vaginal fluid was placed on glass slides, the ingredients were observed under light microscope (YS100, Nikon, Melville, NY) with 40× objective lenses. Three types of cells were recognized: round and nucleated ones as epithelial cells; irregular ones without nucleus as cornified cells; and the little round ones as leukocytes. The proportion among them was used for the determination of the estrous cycle phases.
Total RNA was isolated by using the RNA isoplus (TaKaRa Bio, Shiga, Japan) according to manufacturer’s instruction. After chloroform extraction and isopropyl alcohol precipitation, the final pellet was air dried and dissolved into RNase-free DEPC solution (TaKaRa Bio, Shiga, Japan). The RNA concentration was measured with the Nano-drop (Thermo Fisher Scientific Inc., Waltham, MA). First strand cDNA synthesis was performed in RNase-free DEPC solution containing 2 μg total RNA and 10 pmol oligo dT at 70°C for 5 min, followed by double-strand synthesis in 5× RT buffer (Invitrogen, Carlsbad, CA) with 8 mM dNTP (BIO BASIC INC., Ontario, Canada), 200 unit/μL RTase (Invitrogen, Carlsbad, CA) at 37°C for 60min and at 72°C for 15 min.
Quantitative RT-PCR (qRT-PCR) was performed in a total volume of 20 μL buffer solution containing 2 μL of template cDNA, 10 μL of SYBR Green (Roche, Manheim, Germany), and 10 pmol of each primer (BIONICS, Korea). Primer pairs were as follows: NUCB2 forward 5'-AAAACCTTGGCC TGTCTGAA-3'; reverse 5'-CATCGATAGGAACAGCTT CCA-3', IL-17A forward 5'-TGAGCTTCCCAGATCACA GA-3'; reverse 5'-TCCAGAAGGCCCTCAGACTA-3', and GAPDH forward 5'-TTGATGGCAACAATCTCCAC-3'; reverse 5'-CGTCCCGTAGACAAAATGGT-3' (Bionics, Korea). The optimum temperature cycling protocol was determined to be 95°C for 5 min followed by 45 reaction cycles at 95°C for 10 s, 60°C for 10 s and 72°C for 10 s using the LightCycler® 480 Real-time PCR System (Roche, Manheim, Germany).
The samples were homogenized in ice-cold lysis buffer 50 mM Tris-base (pH 7.4), 150 mM NaCl, 10 mM EDTA, 0.1% Tween-20, and protease inhibitors (0.1 mM phenylmethylsulfonylfluoride, 5 g/mL aprotinin, and 5 g/mL leupeptin). The homogenates were centrifuged at 12,000 × g for 30 min at 4℃. The protein concentration in the supernatant was determined by the BCA protein assay according to the SpectraMax M3 Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA). Equal amounts of proteins (20 μg) were resolved by 12% SDS-PAGE and transferred to PVDF membranes (Amersham; GE Healthcare, Buckinghamshire, England). The membranes were then incubated in blocking solution with 3% Casein Blocking Solution (Komabiotech, Korea) in Tris-buffered saline (TBS) containing 10 mM Tris (pH 7.6), 150 mM NaCl, and 0.1% Tween-20 for 1 h at room temperature. Then, the membranes were incubated with rabbit anti-rat nesfatin-1 polyclonal antibody (H-003- 22, Phoenix Pharmaceuticals, Burlingame, CA) and antimouse β-actin antibody (sc-47778, Santa Cruz Biotechnology, Paso Robles, CA) at 4℃ for overnight. The membrane was washed three times with washing buffer and incubated with donkey anti-rabbit IgG-HRP (sc-2313, Santa Cruz Biotechnology, Paso Robles, CA) and donkey anti-mouse IgG-HRP (sc-2096, Santa Cruz Biotechnology, Paso Robles, CA) at room temperature for 1 h, respectively. After washing three times, the membrane was detected by ECL Plus Western Blotting Detection Reagents (Amersham; GE Healthcare, Buckinghamshire, England). The signal intensity was measured using the LAS-1000 luminescence detector (Fujifilm, Tokyo, Japan).The relative protein levels were analyzed by Image J (National Institutes of Health, Bethesda, MD).
Terminal blood sample were collected from each pregnant mother by cardiac puncture. Serum was obtained by centrifugation of blood samples at 6,000 rpm for 20 min. IL-17A and nesfatin-1 concentration was measured by Mouse IL- 17A (61-ILAMS-E01, Alpoco, Salem, NH) and nesfatin-1 ELISA (FEK-003-22, Phoenix Pharmaceuticals, Burlingame, CA) according to the manufacturer’s instruction. The protein concentration in the serum was determined by the BCA protein assay according to the SpectraMax M3 Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA).
RESULTS
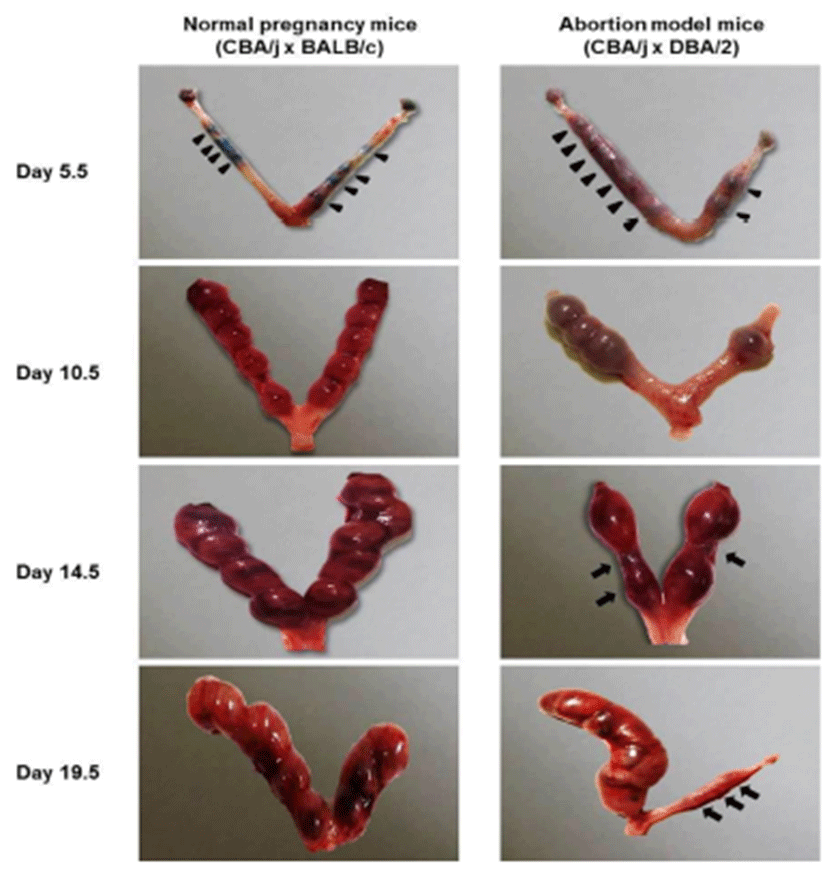
On day 5.5, Chicago blue dye was used to identify the implantation and inter-implantation sites for dissection. Implantation sites (arrow heads) were observed in both normal pregnancy mice and abortion model mice on day 5.5, 10.5, 14.5, and 19.5 of pregnancy, whereas abortion sites (arrows) were found in abortion model mice on day 14.5, and 19.5 of pregnancy (Fig. 1). The uterine of abortion model mice showed less the number of implantation sites compared to normal pregnancy mice.
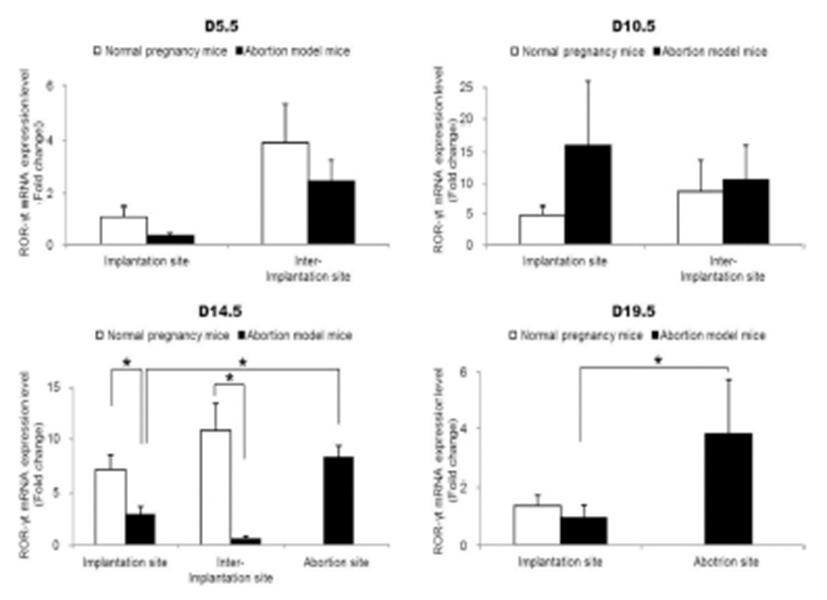
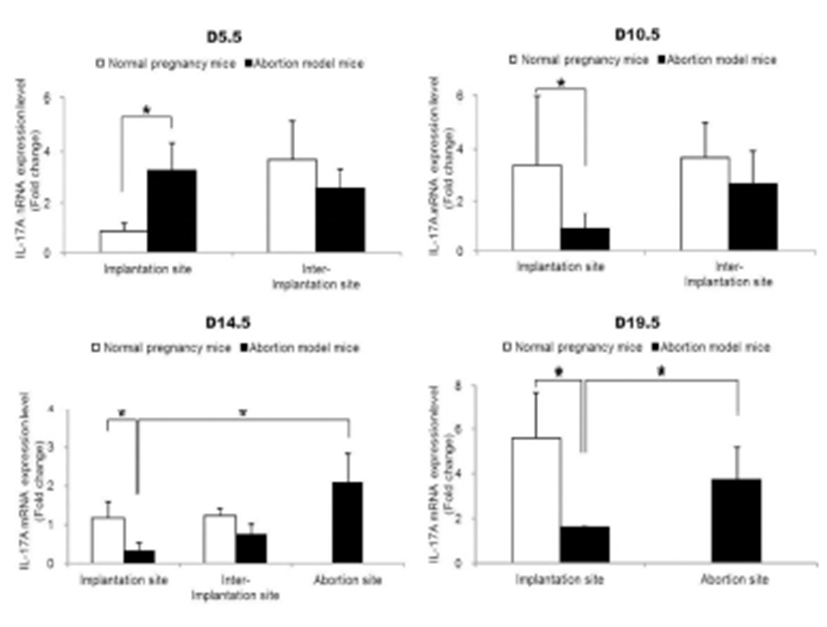
To examine the change of Th17 cells present in implantation sites during the pregnacy, the mRNA expression levels of RORγt as a Th17 cell marker were analyzed by qRT-PCR. Interestingly, Th17 cell transcription factor, ROR-γt mRNA expression levels were significantly increased in the abortion sites compared with the implantation sites of abortion model mice on day 14.5 and 19.5 of pregnancy (Fig. 2). In order to know if these Th17 cells existed in these sites were functioning, the mRNA expression levels of IL-17A which is of cytokines produced by activated Th17 cells were examined by qRT-PCR. The expression levels of IL-17A mRNA were also significantly higher in abortion sites than implantation sites on day 14.5 and 19.5 of pregnancy (Fig. 3).
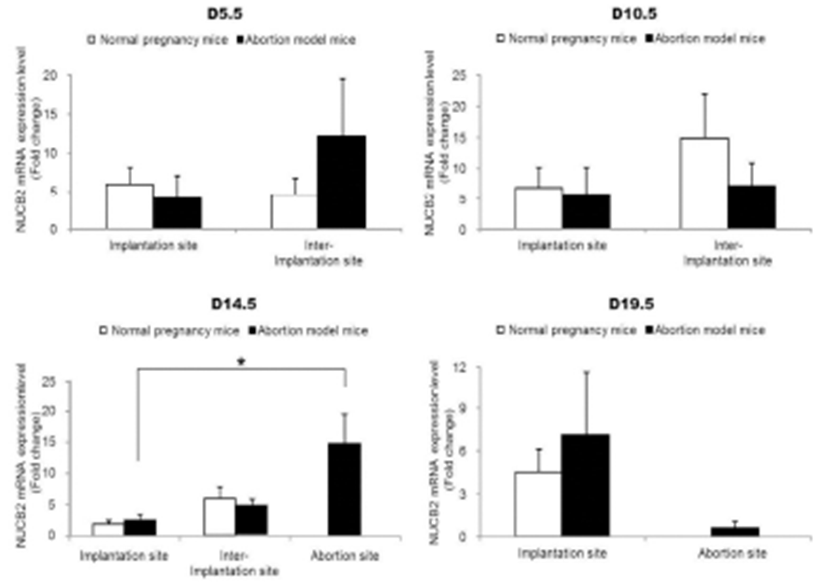
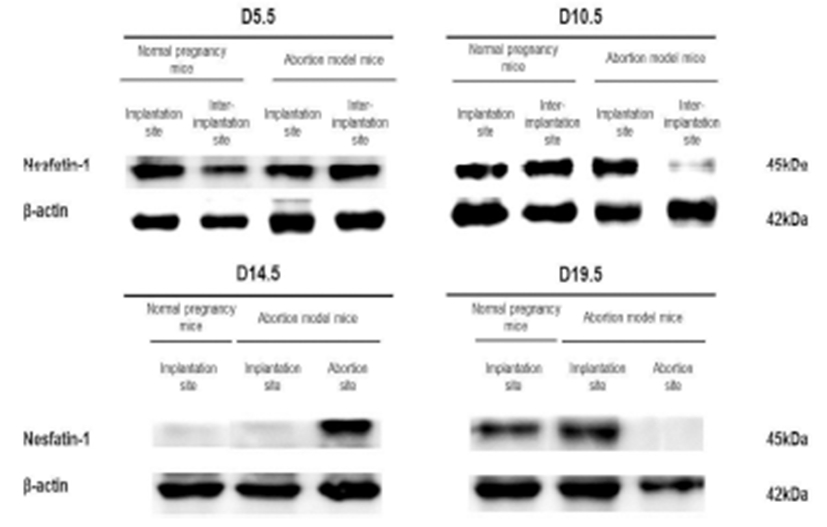
The expression levels of nesfatin-1/NUCB2 mRNA were examined by qRT-PCR in implantation, inter-implantation, and abortion sites during pregnancy. The expression levels of nesfatin-1/NUCB2 mRNA were significantly higher in abortion sites than implantation sites of abortion model mice on day 14.5 of pregnancy (Fig. 4). Interestingly, this change of nesfatin-1/NUCB2 mRNA expression was similar to ROR-γt and IL-17A mRNA expression. However, the nesfatin-1/NUCB2 levels were significantly decreased in abortion sites compared with implantation sites on day 19.5 of pregnancy (Fig. 4). In addition, the levels of nesfatin- 1/NUCB2 protein in implantation, inter-implantation, and abortion sites were investigated by western blotting. As a result, nesfatin-1/NUCB2 protein levels were increased in abortion sites compared with implantation sites of both normal pregnancy mice and abortion model mice, similar to nesfatin-1/NUCB2 mRNA expression (Fig. 5).
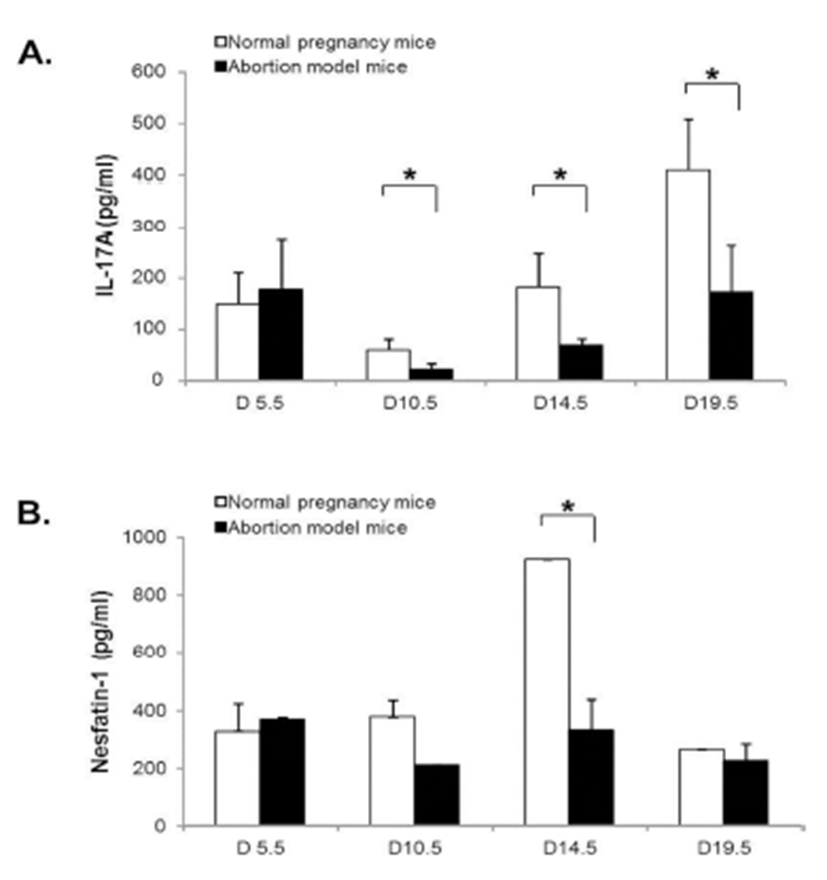
To assess the correlation between nesfatin-1/NUCB2 expression and Th17 cells activation, the serum levels of nesfatin-1/NUCB2 and IL-17A in normal pregnancy mice and abortion model mice during pregnancy were measured using ELISA kit. IL-17A serum levels were significantly decreased in both normal pregnancy mice and abortion model mice on day 10.5, and then increased on day 14.5 and 19.5 of pregnancy. Interestingly, its serum levels were significantly higher in normal pregnancy mice than in abortion model mice after day 10.5 of pregnancy (Fig. 6A). On the other hands, nesfatin-1/NUCB2 serum levels were not changed throughout the whole pregnancy in abortion model mice, whereas the serum levels were dramatically increased on day 14.5, and then rapidly dropped on day 19.5 in normal pregnancy mice (Fig. 6B).
DISCUSSION
The uterus is a part of the common mucosal immune system, sharing structural and functional similarities, and common lymphocyte trafficking networks. The molecular determinants of tolerance and immunity in the reproductive tract are now being identified, and the governing principles are similar to those in other mucosal tissues. Especially, the functional significance of T lymphocytes in the uterine immune response is most clearly demonstrated by their association with pathologies of pregnancy (Robertson, 2000).
Our results demonstrated that Th17 cells, which were expressed ROR-γt as a Th17 cell marker gene, were accumulated in the implantation sites of abortion model mice compared to the normal pregnant mice on day 14.5 of pregnancy. These results indicate that the number of Th17 cells is higher in the uterus of abortion mice than in the normal pregnancy mice before embryo resorption, suggesting the potentially deleterious role of Th17 cells in the pregnancy. Moreover, our results showed that the mRNA expression levels of IL-17A, which is the hallmark cytokine produced by Th17 cells, were also increased in the abortion site on day 14.5 of pregnancy. Previous studies indicated that the decreased levels of regulatory T cells with increased prevalence of Th17 cells and the deregulation of Th17 cells by regulatory T cells are associated with unexplained spontaneous abortion (Wang et al., 2010a, b). In the CBA/j × DBA/2 mouse model which we used, Th17 cells and the related factors, IL-17 and RORγt, were significantly upregulated in abortion mice, and Treg cells and the related factor, Foxp3, were downregulated (Xu et al., 2015). Additionally, intraperitoneal injection of recombinant IL (rIL)-17 induced fetal loss in a normal mouse model, and an anti-IL-17 antibody prevented fetal loss in an abortion mouse model (Xu et al., 2015). Normal pregnant CBA/J mice mated with BALB/c males, which received transvaginal rIL-17, presented with a significantly increased abortion rate compared with the group which received PBS (Wang et al., 2014). In a human study, the number of decidual IL-17+ cells in inevitable abortion cases involving active genital bleeding was significantly higher than that in normal pregnancy cases, suggesting that Th17 cells might be involved in the induction of inflammation in the spontaneous abortion (Nakashima et al., 2010a). The percentage of Treg cells at the implantation window in the unexplained recurrent spontaneous abortion was significantly lower compared with the percentage found in normal non-pregnant women. The percentage of Th17 cells in the former was higher than controls. Expression of IL-23, IL-17, IL-6 cytokines in the former was significantly higher than controls (Saifi et al., 2014). These previous studies are consistent with our results.
In the present study, we showed that nesfatin-1/NUCB2 mRNA and protein were expressed in both the implantation sites and abortion sites during the pregnancy. Our previous studies have also demonstrated that the nesfatin-1 protein was localized on the epithelial cells of the endometrium and uterine glands. Nesfatin-1 binding sites were detected in the epithelial cells of the uterine gland and the neutrophils in the endometrium (Kim & Yang, 2012). Neutrophils are known to play an important role in the collapse and recovery of the endometrium (Kaitu'u-Lino et al., 2007). During the estrous cycle, neutrophils move from the blood into the endometrium while the number of neutrophils is dramatically increased in the estrous phase (Sonoda et al., 1998; Steffl et al., 2010). Moreover, it has been reported that the number of decidual IL-17+ cells was positively correlated with the number of neutrophils in spontaneous abortion cases (Nakashima et al., 2010a). Given these results, the finding that nesfatin-1 binding sites were localized on the neutrophils suggests that the uterine function may be regulated by nesfatin-1 to attract neutrophils from the blood into the endometrium (Kim & Yang, 2012).
In our results, the change of nesfatin-1/NUCB2 mRNA and protein expression was similar to ROR-γt and IL-17A mRNA expression. It is suggested that nesfatin-1/NUCB2 expression in the uteri of implantation and abortion sites may be involved in Th17 cell accumulation and activation. In order to assess the correlation between nesfatin-1 expression and Th17 cells activation, we measured the serum levels of nesfatin-1 and IL-17A in normal pregnancy mice and abortion model mice during pregnancy. Interestingly, IL-17A serum levels were significantly higher in normal pregnancy mice than in abortion model mice after day 10.5 of pregnancy. On the other hand, nesfatin-1 levels in the serum were dramatically increased on day 14.5, and then rapidly decreased on day 19.5 in normal pregnancy mice. Furthermore, nesfatin-1 serum levels were negatively correlated with IL-17A serum levels in both normal pregnancy and abortion model mice (Data not shown). These results indicate that nesfatin-1 in the serum does not affect the movement and activation of Th17 cells in the uterus, whereas locally expressed nesfatin-1 in the uterus may play a role in Th17 cell activation in the implantation and abortion sites.
The present results suggest that Th17 cells in the uterus may play an important role in the period of implantation and for maintenance of pregnancy. Furthermore, the present result provides evidence that Th17 cells in implantation sites may be a key regulator for maintenance of pregnancy and the activation of these cells may be regulated by nesfatin- 1/NUCB2. Further study is needed to elucidate the role of nesfatin-1 expressed in the uterus during pregnancy.
