INTRODUCTION
In fish, light (photoperiod, intensity and spectra) is one of main environmental conditions on the regulation of numerous physiological actions including growth, feeding activity, migration and reproduction (Boeuf & Le Bail, 1999; Migaud et al., 2010; Kim et al., 2013). Studies were conducted to find a suitable light conditions to promote the growth in farmed fish (Simensen et al., 2000; Kissil et al., 2001; Henne & Watanabe, 2003; Taylor et al., 2005). In natural sea water, light spectra were distributed along the depth of water because water column act as a chromatic filter (Villamizar et al., 2011). Generally, red spectrum (long wavelength) is absorbed in the shallow water and blue spectrum (short wavelength) was predominated to deeper depth. According to presence of light wavelength with depth, fish physiological functions and habitat are adapted. However, in general aquaculture system, fish is reared under the shallow water depth and natural light or artificial white light conditions. Therefore, study is required on the appropriate light spectra conditions in fish aquaculture system. Recent studies were noticed that the light spectra affects in somatic growth of teleost fish (Villamizar et al., 2009; Karakatsouli et al., 2010; Hyedamejad et al., 2013). However, these results of fish growth effect by light spectra were always not synchronized and it appeared the species specificity.
The tiger puffer Takifugu rubripes is a commercially valuable species in aquaculture industry of South Korea, China and Japan. However, effect of main environmental condition including light spectra on growth of tiger puffer is no information until now. The aim of the present study is to suggest an effective rearing conditions in tiger puffer aquaculture farm using the ability of light spectra adaptation. We investigate the impact of artificial different light spectra on the somatic growth the rearing tiger puffer and analyze the expression of growth-related genes in each brain and pituitary.
MATERIALS & METHODS
Tiger puffer for the present study were obtained from Tham-Ra Fishery located in Seogwipo, Jeju, South Korea and kept in rearing tank at Marine Science Institute, Jeju National University. Fish were reared under natural photoperiod and water temperature conditions in indoor tank and fed commercial pellets (Daehan co., MP3, Busan, South Korea) twice a day before starting experiment. After sufficiently adapted, fish (n=120, body weight 124.2±1.5 g, total length 18.1±0.1 cm) were divided into three groups; blue (480 nm), green (520 nm) and red (590 nm) wavelength conditions. The light intensity of the blue, green and red were adjusted 150 lx and 15.2±1 ft-cd according to the previous methods (Hur, 2011). Experimental fish were reared during 8 weeks under natural water temperature (10.8–12.5°C) and photoperiod was equated with natural photoperiod using a timer. The fish were fed commercial pellets twice a day during experimental period. Fish body weight (BW) and total length (TL) measured every 4 weeks. In during experimental periods, body weight gain, feed consumption and feed efficiency were calculated. Fish sampling was performed initial five fish and final six fish for each light condition groups. Sampled brain and pituitary tissues were immediately stored –80°C until the analysis.
Collected brain and pituitary tissues of the tiger puffer were absolutely homogenized with RiboExTM (GeneAll, Seoul, Korea) reagent for total RNA extraction and total RNA was extracted following the manufacturer’s protocol. Extracted total RNA with treated using RQ1 RNase-Free DNase (Promega, Madison, WI, USA). RNA concentration measured using the Nano Vue (GEHealthcare, Ver.1.0.1, UK) and 500 ng of total RNA used in cDNA synthesis with PrimeScript RT reagent Kit (Takara Bio Inc, Otsu, Japan).
Synthesized cDNA 20 ng used for Real-time qPCR with Evagreen premix PCR kit (abm Inc, Canada). For real-time qPCR, primer sets of each genes were designed by isolated somatostatins (ss1, ss2 and ss3) and growth hormone (gh) of Takifugu rubripes from National Center for Biotechnology Information (NCBI, Table 1). Real-time qPCR was conducted by CFXTM Real-time System (Bio-Rad, Hercules, CA, USA) and amplification performed on the conditions: initial denaturation at 95°C for 10 min, 40 cycles of 95°C for 15 sec, 60°C for 1 min and last 60°C for 1 min. Growth-related genes expression were normalized to the amount of the internal control ef1-α (elongation factor 1-alpha) gene (Table 1).
RESULTS
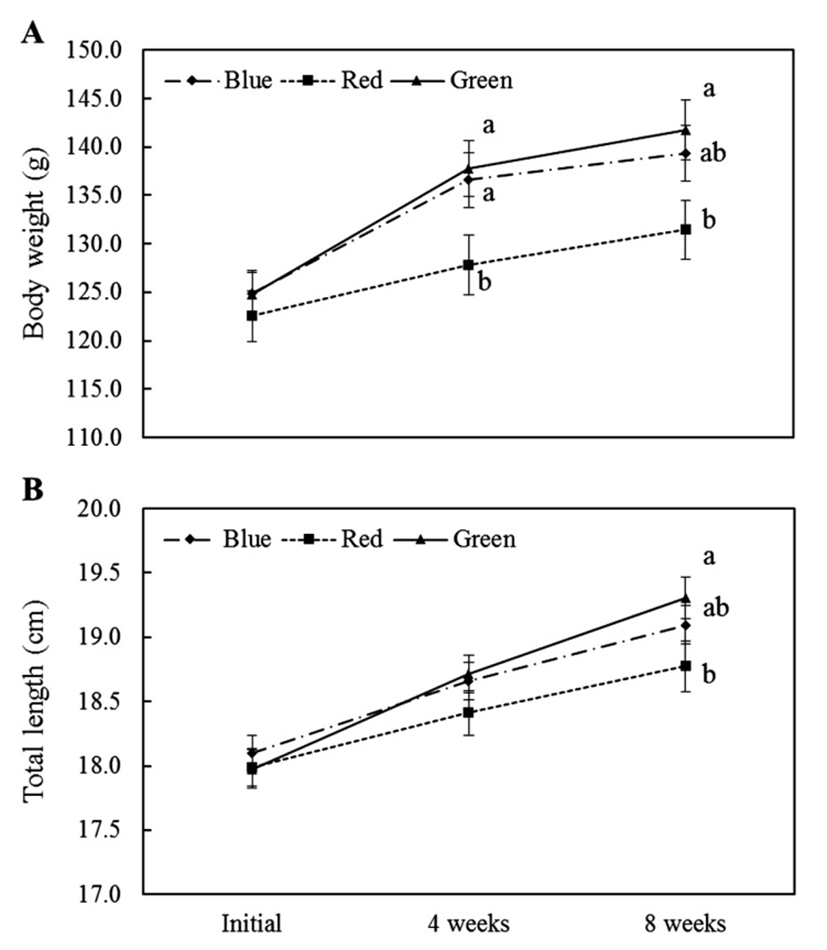
We have measurement of fish BW and TL between experimental groups in every 4 weeks (Fig. 1). Initial fish BW was no different between three groups, but BW of green and blue light conditions groups were more increased than red light condition after 4 weeks. After that, BW of green light condition was steadily maintained significantly more high levels than red light condition until final weeks. The TL of fish was no differed between experimental groups after 4 weeks, but in final weeks, TL of green light condition was significantly increased than red light condition. In during experimental period, change of survival rate, weight gain and feed efficiency were shown in Table 2. The fish in each light condition groups did not die during the experiment. The fish of green light condition group was showed more high weight gain and feed efficiency than other light condition groups.
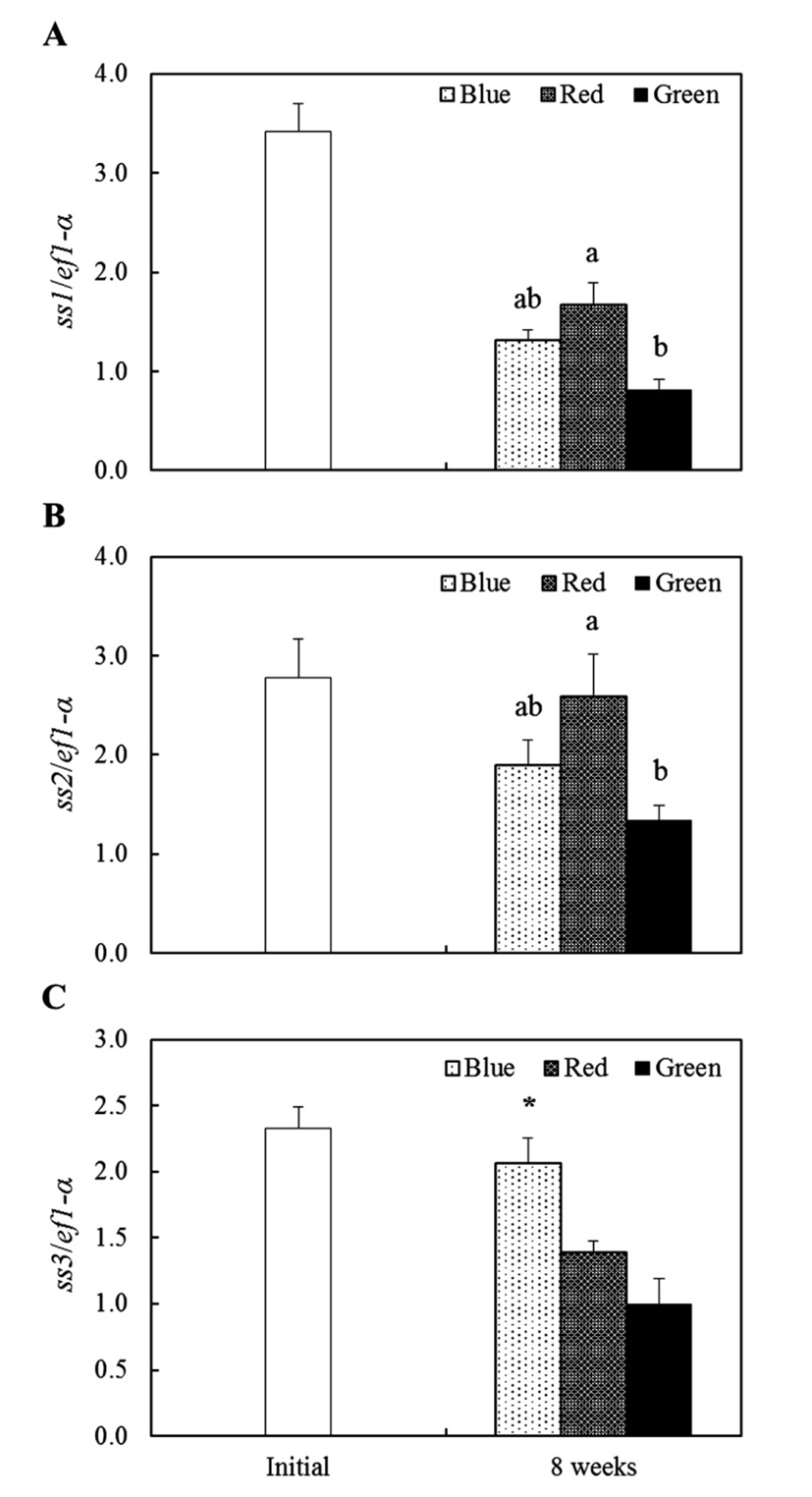
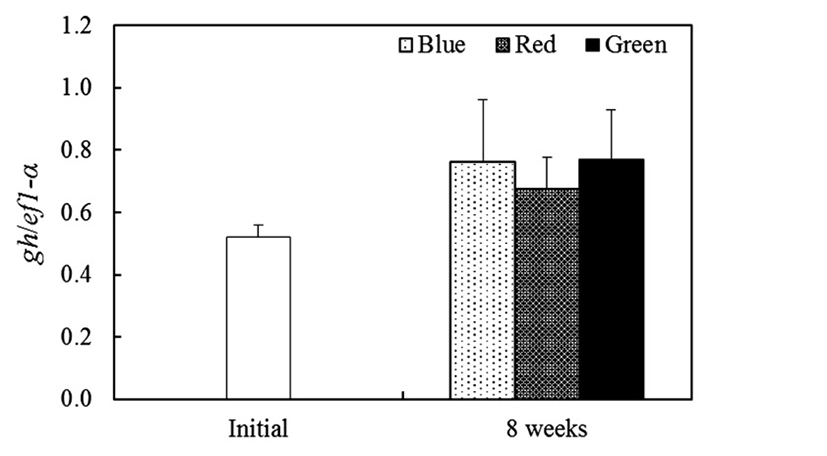
We profiled somatostatins (ss1, ss2 and ss3) and growth hormone (gh) mRNA expression conducted by different light wavelength in tiger puffer. After 8 weeks, the ss1 mRNA of red light condition was shown significantly (P<0.05) high expression than other experimental groups and ss1 mRNA expression of green light condition was lowest than other groups at 8 weeks (Fig. 2A). The ss2 mRNA was showed similar expression pattern with ss1 mRNA and red light condition was observed higher expression than other groups at 8 weeks (Fig. 2B). The ss3 mRNA was observed more increased expression in blue light condition than other experimental groups at 8 weeks (Fig. 2C). In pituitary, gh mRNA was showed no specific expression between the three groups at 8 weeks (Fig. 3).
DISCUSSION
Recently, characteristics of adaptive physiology of fish in rearing environmental factors apply to aquaculture. We discuss about information of adaptive physiology of fish in light spectra. Effect of light spectra on the physiological actions (growth, feeding, stress response and reproduction) have been studied in teleost fish (Volpato & Barreto, 2001; Blanco-Vives et al., 2010; Bapary et al., 2011; Volpato et al., 2013). Especially, effect of light spectra on the somatic growth were announced from some fish. For instance, BW and TL of barfin flounder were increased when reared under green or blue (short wavelength) light conditions and fish growth of red group was remain behind than other groups (Yamanome et al., 2009). Also, green or blue wavelength was reported to the positive effect on the fish growth via haddock (Downing, 2002), crucian carp, Chinese sleeper (Ruchin, 2004), olive flounder (Ko & Park, 2012) and rock fish (Shin et al., 2015). Based on these results, we performed study of light spectra effect on the growth of tiger puffer. Tiger puffer was reared in different light conditions (blue, green and red) for 8 weeks and showed better somatic growth when reared under green light condition. However, reared tiger puffer under red light condition was shown slower growth than other experimental groups. In contrast to our results, rainbow trout showed negative impact on growth performance when rearing under blue light condition (Karakatsouli et al., 2007) and yellow light was efficiently induced the mean weight (Heydarnejad et al., 2013). These different results were suggest that the light spectra represents a species specific effect in fish growth, because preferred wavelength was different for each fish species.
Nevertheless, physiological mechanism of light spectra with growth of fish is still unclear and hardly study. Therefore we investigated changes of weight gain, feed efficiency and growth-related genes expression (ss1, ss2, and ss3) under different light spectra conditions. In generally, SS is well known growth hormone-inhibiting hormone (GHIH) and released in hypothalamus (Sheridan & Kittilson, 2004; Klein & Sheridan, 2008). This hormone is inhibited GH secretion in pituitary gland and shows a negative effect on the growth in goldfish (Cook et al., 1984) and orange-spotted grouper (Wang et al., 2014). Furthermore, SS is related to feeding behavior and digestion in fish. In rainbow trout, food conversion was significantly reduced by SS implantation (Very et al., 2001). In contrast with SS, GH is released hormone in pituitary and it has ability of somatic growth control with many biological actions including appetite, osmoregulation and reproduction in teleost fish (Tatsuta & Hirano, 1993; Mingarro et al., 2002; Kim et al., 2015). In our presents, weight gain and feed efficiency were higher in green light condition, but in red light condition weight gain and feed efficiency were low. The ss1 and ss2 genes of brain were showed high expression when reared under red light condition and its expression were low when reared under green light condition. Therefore, we present results suggest that light spectra were acts as controller of ss gene expression regulation and this ss gene expression will have an effect on the feeding behavior and digestion in fish. However, retardation of gh expression in pituitary was not observed and showed no different expression between experimental groups. In different light conditions (blue, green and red), yellowtail clownfish gh mRNA expression was more higher under green and blue light conditions than under red light condition and induced higher growth rate (Shin et al., 2012). These data were indicate that light spectra affects in endocrine system associated with growth. Although, our present was not confirmed difference of gh expression in pituitary, but gh expression in red light condition was tended to lower than blue and green light conditions. We suggest that ss genes are affect directly or indirectly on the gh expression and further studies are required to relationship of ss with gh genes in tiger puffer.
In this study, green light condition induces higher somatic growth and reducing expression ss1 and ss2 in the brain, but, red light condition is conflicting results with somatic growth and ss1 and ss2 expression. It suggest that red wavelength affects in growth retardation by promoting activation of ss expression. Therefore, artificial green light wavelength is suggest more effective on the growth stimulation than red light wavelength in tiger puffer aqua-farm system, but further studies are needed to clarify the mechanism of light spectra with growth endocrine system.
