INTRODUCTION
Neptunea (Barbitonia) cumingii (Gastropoda: Buccinidae) is one of commercially important edible gastropods in East Asian countries such as in Korea, Japan, China, and Russia. This species is especially found in the subtidal zone of the west coasts of Korea (Yoo, 1976; Kwon et al., 1993). Recently, as the standing stock of this species gradually decreased due to extensive reclamation projects and overharvesting, it has been designated as one of the important organisms in need of natural resources management. For the propagation and management of a living natural resources, it is important to understand its population characteristics with regard to spermatogensis and testicular development.
Previously there have been some studies on aspects of reproduction of Neptunea spp., including the reproductive cycle (Takahashi et al, 1972; Takamaru & Fuji, 1981; Fujinaga, 1985; Kawai et al., 1994) and spawning (Miyawaki, 1953; Amio, 1963; Son, 2003) of this species, on ecology, including the distribution(Ito & Tachizawa, 1981; Ito, 1982) and growth (MacIntosh & Paul, 1977; Fujinaga, 1987; Suzuki et al., 1996) of N. arthritica. and the feeding of N. antigua (Pearce & Thorson, 1967). However, little information is avaiable on the ultrastructure of germ cells during spermatogenesis and structural changes in epithelial cells of the seminal vesicle with testicular development of N. (B.) cumingii. Therefore, the purpose of the present study is to understand ultrastructures of germ cells and spermatogenic stages and the structural changes and the functions in the epithelial cells of the seminal vesicles in relation to the gonadal phases, using cytological and histochemical procedures.
MATERIALS AND METHODS
Specimens of Neptune whelk, N. (B.) cumingii Crosse, 1862 (Buccinidae) were collected monthly by dredge at the subtidal zone in the vicinity of Piung-do, Korea, from June 2010 to May 2011 (Fig. 1). 256 Neptune whelks ranging from 8.1-11.2 cm in shell height were used for the study. After the neptune whelks were transported alive to the laboratory, shell heights were measured by a Vernier caliper, and total weights were weighed using a chemical balance.
For light microscopic examination of histological preparations (seminal vesicles), a total of 210 male individuals were used for histological analysis of the gonads from June 2010 to May 2011. Gonad tissues were removed from shells and preserved in Bouin’s fixative for 24h and then washed with running tap water for 24h and then washed with running tap water for 24h. Tissues were then dehydrated in alcohol and embedded in paraffin molds. Embedded tissues were sectioned at 5-7 μm thickness using a rotary microtome. Sections were mounted on glass slides, stained with Hansen’s hematoxylin-0.5% eosin, Mallory’s triple stain and PAS stain, and examined using a light microscope.
For transmission electron microscope observations, a total of 60 male individuals were used for spermatogenesis, excised pieces of the testes were cut into small pieces and fixed immediately in 2.5% paraformaldehyde-glutaraldehyde in 0.1 M phosphate buffer solution (pH 7.4) for 2 hours at 4°C after prefixation, the specimens were washed several times in the buffer solution and then postfixed in a 1% osmium tetroxide solution in 0.2 M phosphate buffer(pH 7.4) for 1 hour at 4°C Specimens were then dehydrated in increasing concentrations of ethanol, cleared in propylene oxide and embedded in an Epon-Araldite mixture. Ultrathin sections of Epon-embedded specimens were cut with glass knives on a Sorvall MT-2 microtome and LKB ultramicrotome at a thickness of approximately 80-100 nm. Tissue sections were mounted on collodion-coated copper grids, doubly stained with uranyl acetate followed by lead citrate, and observed with a JEM 100 CX-II 80-KV) electron microscope.
RESULTS
Male individuals of N. (B.) cumingii can be distinguishable easily by existence of the penis, which occurs near the tentacles. The testis is located on the surface of the liver which is located in the posterior spiral visceral mass. With the progression of maturation, the external feature of the testis appears yellowish-brown in colour. If the testis is slightly scratched, milky white sperm readily flow out.
Based on germ cell development and morphological characteristics as seen with the electron microscope, spermatogenesis in the testicular lobules can be classified into five stages: (1) spermatogonial, (2) primary spermatocyte, (3) secondary spermatocyte, (4) spermatid, and (5) spermatozoon.
Spermatogonia are located at the periphery of the spermatogenic follicle, and are about 9 μm in diameter and more or less oval in shape. The spermatogonia contain a large nucleus, and relatively small cytoplasm. The nucleus contains dense and unevenly distributed heterochromatin. The Golgi apparatus, several mitochondria and small dense granular materials are distributed in the cytoplasm (Fig. 2A).
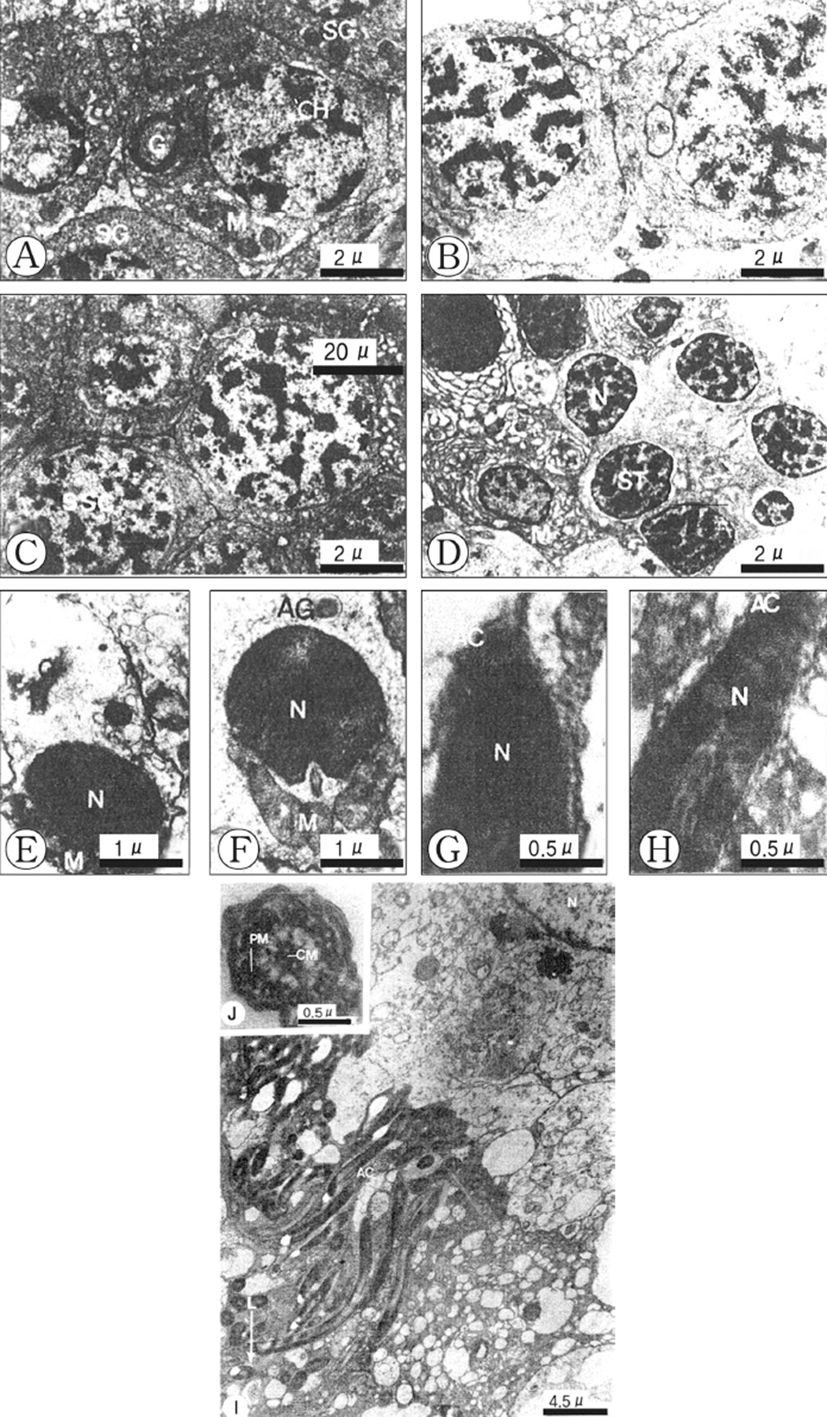
Spermatogonia develop into the primary spermatocytes, which are smaller (about 7 μm in diameter) than spermatogonia. Their nuclei contain slightly denser heterochromatin materials. Synaptonemal complexes in the nucleus appear during the prophase of meiosis. A few mitochondria can be seen tin the cytoplasm of the cell (Fig. 2B).
The primary spermatocytes develop into the secondary spermatocytes by way of the first maturation division. Secondary spermatocytes are about 6 μm in diameter and their nuclei are coradensed with more dense heterochromatin materials. At this time, a few mitochondria, endoplasmic reticula and small vacuoles are present in the cytoplasm (Fig. 2C).
The secondary spermatocytes develop into spermatids through the second maturation division, which have the typical structure of an interphase nucleus with aggregated chromatin. By this time the peripheral parts of the cells have many smooth membrane configurations in the form of flattened vesicles, small mitochondria are elongated (Fig. 2D).
Early in differentiation, the shape of the condensed nucleus becomes slightly elongate. The Golgi apparatus and acrosomal granule, which is located near the nucleus, move to a position just before the nucleus, while the mitochondria and centrosome move to a position just behind the nucleus of the spermatid (Figs. 2E, F). During a later developmental stage of spermiogenesis, the acrosomal vesicle becomes closely applied to the tip of the elongating nucleus. The acrosomal granule in the acrosomal vesicle forms the core of the acrosome (Fig. 2G). Afterward, the shape of the acrosomal vesicle changes to an elongated acrosome, and then it changes to a cap-shape. The nucleus of the spermatozoon becomes longer, the head of the spermatozoon becomes elongate, and the axial filament enters into the long nucleus of the spermatozoon longitudinally (Fig. 2H).
A ripe spermatozoon is approximately 50 μm in length (Fig. 3I). At this stage, the centrioles gave rise to the axial filament of the flagellum of the spermatozoon and sperm morphology showed the modified type, as found in internal fertilization species. After acrosome formation is completed, the promonent nuclear canal in the nucleus in present in cross-sections of the sperm head. The sperm head is ca. 5 μm long, including the 0.2 μm long acrosome, and its tail is ca. 20 μm long (Fig. 2I). The axoneme of the tail flagellum is composed of nine pairs of microtubules at the periphery (Peripheral mictotubules) and one pair of cental microtbubules (Fig. 2J).
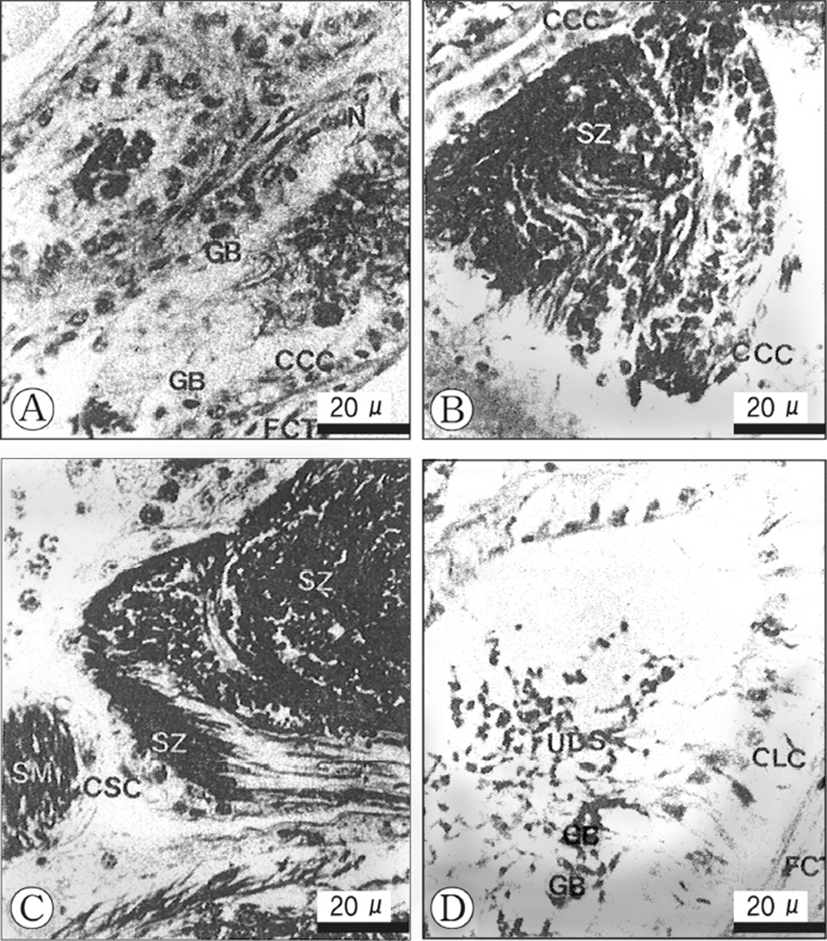
Histological changes in the epithelial cells according to developmental phases of the seminal vesicles Based on histological/morphological characteristics of the epithelial cells of the seminal vesicle wall, viz., the convoluted part of the vas deferens, and the relative amount of spermatozoa accumulated in the lumina of the vesicles, morphological changes of seminal vesicles could be divided into three phases: (1) S-I phase (resting), (2) S-II phase (early accumulating), and (3) S-III phase (active accumulating), and (4) S-IV (spent). These succesive phases show a periodicity. The criteria used in defining the categories are as follows.
The fibrous connective tissues, which comprise the vas deferens, show several thick layers, but are somewhat contracted. The epithelial cells of the inner layer of the seminal vesicle are composed of ciliates cuboidal cells of a single layer, and have oval or conical nuclei which are stained darkly with haematoxylin. At this phase, a few granular bodies appear in the cytoplasm of the epithelial cells in the inner layer. In particular, At this phase, a few yellow granular bodies appear in the cytoplasm of the epithelial cells in the inner layer of the seminal vesicle (Fig. 3A). The resting phase of the seminal vesicle appear during the periods when the developmental stages of the testis are in the recovery and the early active stages.
In the early accumulating phase of the seminal vesicle, the epithelial cells change to cuboidal cells with round nuclei and clear vacuoles in the cytoplasm, but yellow granular bodies begin to disappear (Fig. 3B). Subsequently, as the sperm masses produced in the testicular lobules, they enter the lumina of the seminal vesicle, the vesicle become milky white gradually. Thereafter, with the input of the sperm masses into the vesicles, At this phase, small number of sperm masses are present in the lumen of the vesicle.
In the late accumulating, a number of sperm masses are present in the lumina of the vesicles. The cuboidal cells in the epithelium of the seminal vesicl change into squamous cells (10 Each squamous cell has an elongate nucleus and the connective tissues in the outer layer of the vesicle become thin (Fig. 3C). The active accumululating phase of the seminal vesicle continued during the ripe stage in the testicular lobules. Their cells become greater in height (Fig. 3C). This phase is found during the period of large number of sperms masses are present in the lumina of the seminal vesicles. At this time, granular bodies disappeared during sperm storage.
In this phase, a number of discharged sperm masses are still present in the seminal vesicles. The ciliated epithelial cells of the inner layer change into columnar cells, which become taller. Furthermore, the fibrous connective tissues of the outer layer increase in thickness remarkably. This phase was found during the period of copulation in the testicular lobules. At this phase, a few yellow granular bodies appeared clearly in the cytoplasm of the inne layer of the seminal vesicle (Fig. 3D).
DISCUSSION
Compared with most marine neogastropods, spermatogenesis of N. (B.) cumingii is very similar to that of other neogastropods with internal fertilization. The acrosome shape of molluscan sperms can be classified into four types (Kim et al., 2010; Kim & Kim, 2011; Kang et al., 2012; Son et al., 2014): cone, cap, elongate-modified cone, and modified cap types. Moreover, sperm nucleus types vary with molluscan species. The sperm nuclei are cylindrical in Septifer virgatus and some Mactra, ovoid in the Ostridae or modified cylindrical in the Veneridae; Jar-shaped in Solen and Corbicular japonica. In N.(B.) cumingii, the acrosome is the cone type. Based on morphological features of gonadal development and on histological observations of the male genital ducts, a number of sperm masses are formed in the lumina of testicular lobules and are accumulated in the semial vesicles during most gonadal phases, except for degeneration of a few stage. It is assumed that male individuals pass Winter with large quantities of sperm masses in the seminal vesicles, and wait for the time of copulation between the spring and summer seasons. In general, breeding-havits of molluscs can be classified into three large categories: (1) year-round breeders, (2) winter breeders and (3) summer breeders (Boolootian et al., 1962). According to the results of our histological study, N. (B.) cumingii belongs to the summer breeder class. Most shallow-water marine animals reproduce in a cyclic manner, the time of spawning ultimately depending on environmental factors (Kinne, 1963; Brousseau, 1978). In the most commonly studied gastropods, the time of copulation is linked to water temperatures (Webber & Giese, 1969; Chung et al., 1993). Some local variations of the copulation period of this species might be related to the geographical differences in food supply and feeding (Amio, 1963). In the case of species with internal fertilization, it is especially hard to estimate exactly the copulating period by only observing copulation behaviour under natural conditions. Therefore, the copulating period should be investigated by histoloigical and cytological studies (Takahashi et al., 1972). In mesogastropods and neogastropods, it is well known that the spawning of female individuals commonly occurs after copulation (e.g., Amio, 1963; Feare, 1970; Takahashi et al., 1972; Fujinaga, 1985). Fujinaga (1985) reported that the time of copulation occurring before spawning varies with the species. In gastropods, the times of copulation were just before spawning in Nucella lapillus (Feare, 1970) and Purpura clavigera (Amio, 1963), and over 10 days in Neptunea arthritica (Fujinaga, 1985). In the present study, the copulating period of the male N. (B.) cumingii was from April to July, however and the spawning period in females was from May to August. Therefore, it is assumed that the interval between the copulating period of males and the spawning period of females in this species is longer than those of the other species. And also the copulation period of the male R.venosa was from April to July. Therefore, it is assumed that the interval between the copulating period of males and the spawning period of females in R. venosa longer than those of the other species. Takahashi et al. (1972), and Fujunaga (1985) described the structural changes and functions of the genital ducts. Regarding cytological changes in the epithelial cells of the seminal vesicles of N.(B.) arthritica cumimingii, Chung et al. (2005) reported that the inner layer’s epithelial cells showed remarkable morphological changes with various gonadal stages. Especially, in the resting stage before sperm accumulation, yellow granular bodies were shown in the epithelial cell layer, and then these bodies disappeared during sperm storage. However, they appeared again during the spent stage. In case of N. (B.) cumingii, histological changes in the epithelial cells of the seminal vesicles showed some similar phenomena; histological changes in the epithelial cells of the inner layers of the seminal vesicle related to the spermatogenic stages during spermatogenesis in the testicular lobules showed very similar characteristics. Regarding the developmental phases of the seminal vesicle of N. (B.) arthritica cumimingii, Chung et al. (2005) reported three phases: resting, accumulating, spent phases. However, in case of N. (B.) cumimingii, the developmental phases of the seminal vesicle can be divided into four phases: (1) the resting phase, (2) the early accumulating phase, (3) the active accumulating phase, and (4) the spent phase. The morphological differences of the epitheial cells between two species (N. (B.) arthritica cumimingii and N. (B.) cumimingii) showed somewhat differents, however, in general, their structural changes in accordance with the developmental phases of the seminal vesicle showed very similar patterns.
Fretter (1941) reported that yellow granular bodies in the cytoplasm of the inner layer’s epithelial cells in Baresinum undatum and N. lapillus play an important role in absorption and digestion of residual sperm. Chung et al. (2005) and Takahashi et al. (1972) reported that yellow granular bodies in the cytoplasm of epithelial cells of the seminal vesicle of N. arthritica have a lysosome-like structure. In the present study, yellow granular bodies in the cytoplasm of epithelial cells of the seminal vesicle appeared during the resting phase and the spent stage and we found results similar to those of Fretter (1941), Takahashi et al. (1972) and Chung et al. (2005) and I came to similar conclusions. It is assumed that granular bodies are involved in resorption of digestion of residual spermatozoa. Further study by electron microscope is needed on the granular bodies structure, functions and their fate.