INTRODUCTION
The far eastern catfish, Silurus asotus and the slender catfish, Silurus microdorsalis belonging to the Siluridae are only two species that inhabit in Korea (Mori, 1936; Jeon, 1984). S. asotus prefer to dwell in the muddy floor of rivers and lakes and distributed in Korea, Japan, Taiwan, and China. On the other hand, S. microdorsalis is an endemic species in Korea which is found in only mountainous streams (Park & Kim, 2005).
S. asotus is one of the most important fresh water aquaculture species in Korea. It produces 4,764 tons in 2014, 4,267 tons in 2015, and 4,953 tons in 2016 (National Statistics Office Database). S. microdorsalis has attracted a lot of attention as a promising species, but until now only a small number of seedlings has produced at the regional research institute.
The released young fish will grow to breeding groups to make offspring, which in turn increase the population and restore the resources (Jeong & Jeon, 2008). The restoration of fish resources can only be achieved by improving the physical environment of the habitat, recovering the damaged habitat, and restoring the fish population together. For the conservation of endangered species, it is very important to clarify the reproductive characteristics and to produce artificial seedlings. A few studies have been reported on slender catfish: Comparison of carotenoid pigments in catfish, Parasilurus asotus and slender catfish, Parasilurus microdorsalis in the family siluridae (Baek et al., 2004), Comparison of carotenoid pigments in catfish, Parasilurus asotus and slender catfish, Parasilurus microdorsalis in the family siluridae (Park et al., 2004), Comparison of ultrastructure of spermatozoa of two catfish, Silurus asotus and S. microdorsalis (Kwon & Kim, 2004), and osteological study of Silurus microdorsarlis (Lee & Kim, 1987).
In present study, we aimed to explore the reproductive cycle of Silurus microdorsarlis, a Korean endemic species, to lay grounds for species conservation, genetic resource procurement and the restoration of mountainous river ecosystem.
MATERIALS AND METHODS
A total of 10 female and male Silurus microdorsalis were collected monthly from Sueocheon tributary near Jinsangmyun, Gwangyangsi, Jeollanamdo (35°01’21”N, 127° 43’12”E) during January to December 2013 using weir traps and skimming nets. Collected samples were delivered to the laboratory facility alive, to dissected carefully.
Water temperature was measured using digital thermometer during sampling. For day length, we recorded and converted the day length of every 15th on Suncheon Meteorological Observatory data.
Samples were measured until 0.1 cm unit scale for length and 0.01 g unit scale for weight, then the abdomen was dissected to weigh the gonads until 0.001 g unit scale.
We calculated the gonadosomatic index (GSI) from N’ Da & Deniel method (1993) (gonad weight / body weight x 100) in order to examine the monthly variation in gonadal maturation status.
Excavated gonads were fixed for 24 hours in Bouin’s solution (picric acid:formalin:acetic acid = 15:5:1), embedded in paraffin and sliced into 5-7 μm of serial sections, and then double-stained with hematoxylin and eosin. The female reproductive cycles was defined by follicular development stages in the ovaries, while male reproductive cycle was defined by progress of spermatogenesis stages in the testis.
RESULTS
The habitat’s water temperature, day length, and the GSI of slender catfish are described in Fig. 1. From December to February, the GSI was 3.90±1.79, 4.68±1.74 and 4.70± 1.18 for female and 0.56±0.19, 0.59±0.12 and 0.68±0.16 for male, respectively. The range of water temperature was 5-7°C during this period. The water temperature increased from March (12°C) to August (28°C), the GSI value began to increase to in March and reach to 11.77±3.23 for female and 1.23±0.33 for male in May (21.5°C). After then, the GSI value decreased gradually for both female and male to 8.02±1.99, 6.67±0.17, 5.24±0.72, and 0.93±0.38, 0.63± 0.10, 0.76±0.15 in June (24°C), July (26.5°C), and August (28°C), respectively. From September (25°C) to November (13°C), water temperature decreased gradually, and the GSI value was 1.39±0.12, 1.36±0.08, 3.12±0.25 for female and 0.76± 0.05, 0.62±0.10, 0.64±0.09 for male, respectively.
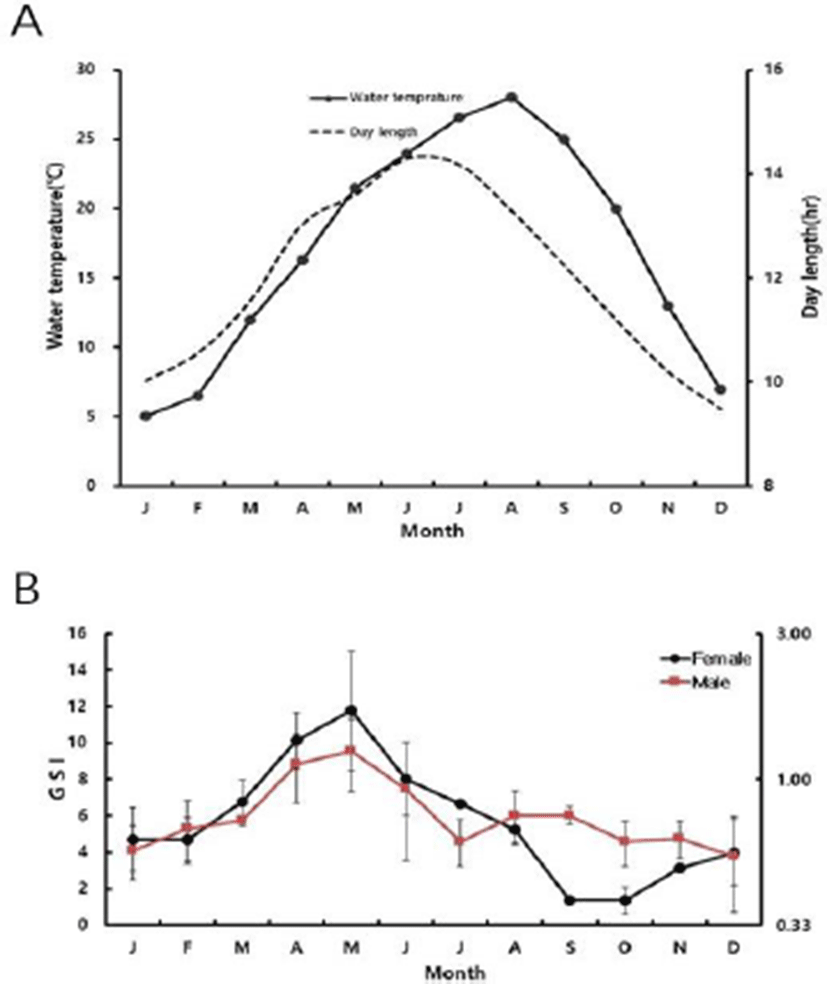
Regarding day length, it was 9.49 hr in December (the shortest of the year) and gradually lengthened to 10.03 hr, 10.56 hr, 11.56 hr, 13.03 hr 13.59 hr, 14.29 hr in January, February, March, April, May, and June (reaching the longest). Subsequently it decreased to 14.17 hr in July, 13.29 hr in August, 12.25 hr in September, 11.2 hr in October, and 10.2 hr in November.
Development stages of oocyte and follicles were histologically examined (Fig. 2) and calculated as frequencies. The female reproductive cycle were classified according to Elorduy-Garay & Ramirez-Luna method (1994) as the growing phase, the mature phase, the ripe and spawning phase, the degenerative phase, and the resting phase (Fig. 4).
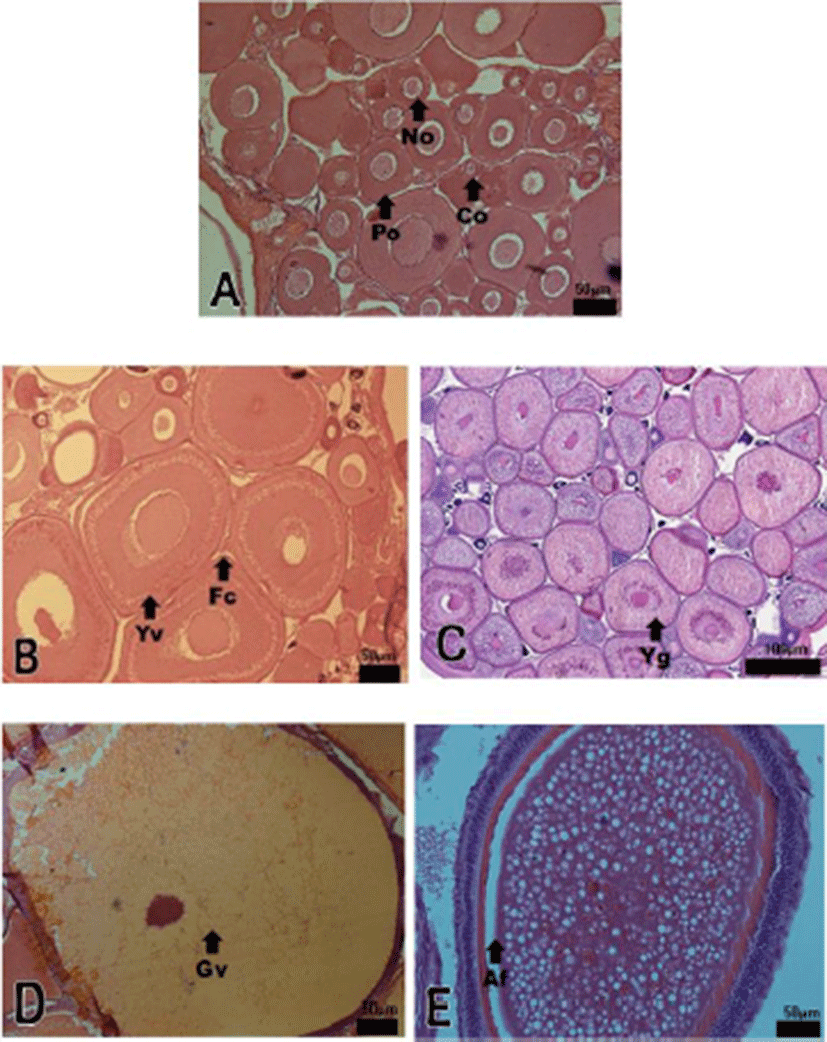
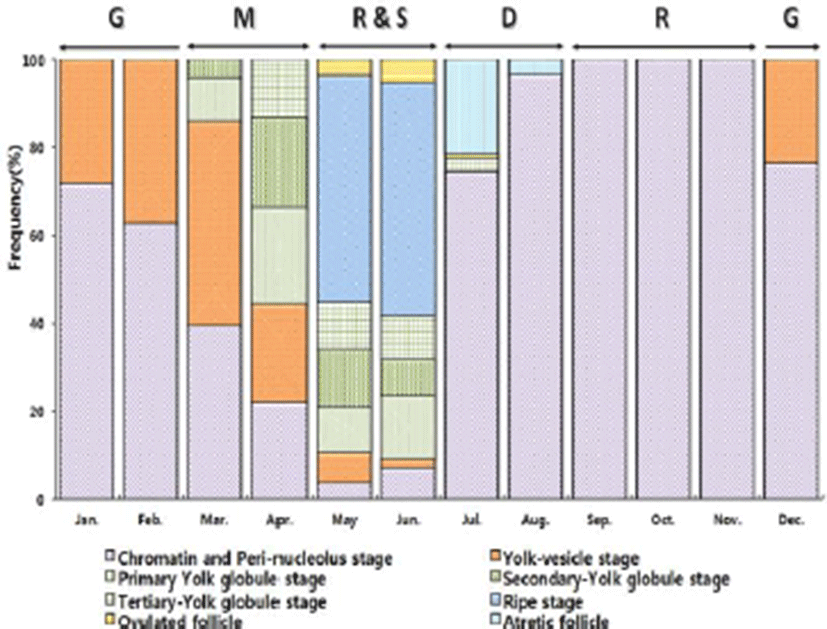
The growing stages oocytes (chromatin nucleolus and peri-nucleolus, and yolk vesicle) were 76.54% and 23.46% in December, 71.69% and 28.31% in January, 62.85% and 37.15% in February.
The maturing stage oocytes (primary yolk globule, secondary yolk globule and tertiary yolk globule) were appeared from March to April. In March, the frequency of primary-tertiary yolk globule stage oocytes was 55.51%.
The ripe (hydrated) stage oocytes appeared from May to June, In May, the frequency of ripe stage oocytes and ovulated follicles was 51.58% and 3.63%, respectively. In June, the frequency of ripe stage oocyte and ovulation follicle was 53.18% % and 5.24%, respectively.
From July to August, atretic follicles appeared in the ovary, July-August could be classified as the degenerative phase. The resting stage oocytes (chromatin nucleolus and peri-nucleolus, and yolk vesicle) appeared from September to November.
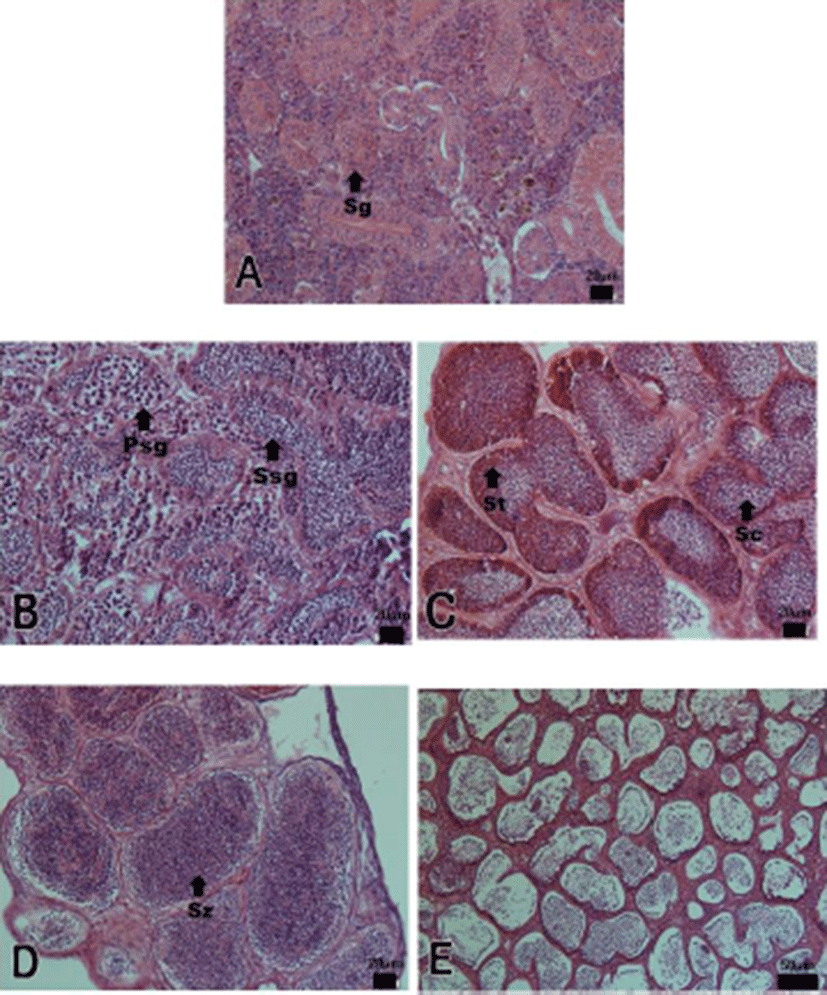
The progress of spermatogenesis stages were histological examined (Fig. 3), calculated as frequencies. The male reproductive cycle were classified as the growing, the mature, the ripe and releasing, the degenerative, and the resting phase (and Fig. 5).
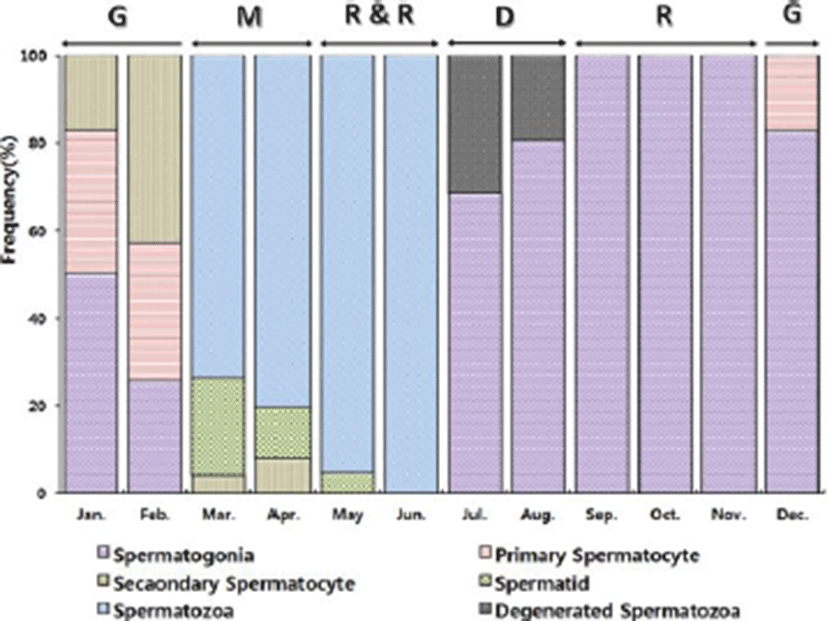
The growing phase (spermatogonia and spermatocyte) of male was from December to February. The frequency of spermatocyte was 17.35% in December, 49.69% in January and 74.04% in February.
The mature phase (spermatocyte, spermatid and spermatozoa) of male was from March to April. The frequency of spermatozoa was 73.65% in March and 80.32% in April.
The ripe and releasing phase (seminiferous tubule of testes with spermatozoa) was from May to June. The degenerative phase was from July to August. A small number of sperm remained in the lumen of seminiferous tubules, after ejaculation. The resting phase (spermatogonia proliferation) was from September to November.
DISCUSSION
The reproductive cycle of fish is determined by the endocrine system of the hypothalamus-pituitary-gonadal (HPG) axis, which is influenced remarkably by external environmental factors such as water temperature and photoperiod (Vlaming, 1972; Breton et al., 1977; Crim, 1982; Lam, 1983; Razani et al., 1988; Aida, 1991; Shimizu et al., 1994). The fish inhabit the mid-latitudes spawn at the time when their offspring are more likely to survive, and this spawning season repeats every year (Lee et al., 1984). The spawning season, the culmination of the reproductive cycle, is the time when mature eggs and sperm meet to form fertilized eggs that will carry a new generation. Studies on the reproductive cycle of freshwater fish have been conducted to conserve species and production of seeds. There are few comparative studies among major species inhabiting the same water system.
Siluriformes inhabiting major water systems in Korea are the slender catfish Silurus microdorsalis, the South torrent catfish Liobagrus mediadiposalis, the Black bullhead Pseudobagrus koreanus, the bullhead Pseudobagrus fulvidraco and the far eastern Catfish Silurus asotus. Among siluriformes the slender catfish, the South torrent catfish and the Black bullhead are Korean endemic species that live only in Korea (Kim, 1997).
Slender catfish live mountainous stream. South torrent catfish habitats in overlap with slender catfish, but live in a wide area. Black bullhead distribute in the upstream of the main river.
Currently the reproductive cycle of South torrent catfish and Black bullhead are already reported, but not slender catfish.
The reproductive cycles of siluriforms such as South torrent catfish, Black bullhead, bullhead studied to date and has been reported five reproductive stages involving growing phase, mature phase, ripe and spawning phase (releasing for male), degenerative phase, and resting phase (Lim & Han, 1997; Hwang, 2006; Choi, 2008). The reproductive cycle of slender catfish also showed five stages of growing, mature, ripe and spawning (releasing for male), degenerative, and resting phase.
However the timing and duration of each stages could differ from species to species. South torrent catfish exhibit growing phase in January-February, mature phase in March- April, ripe and spawning (releasing for male) phase in May-June, degenerative phase in July-August, and resting phase in September-December (Choi, 2008). Black bullhead also demonstrates growing phase (March-April), mature phase (May), ripe and spawning phase (June-July), degenerative phase (August-September), and resting phase (October-February). On the other hand, bullhead exhibits growing phase (April-early May), mature phase (May-early June), ripe and spawning phase (June-August), degenerative phase (September-November), and resting phase (December-March). In Our study, slender catfish represents growing phase (December-February), mature phase (March-April), ripe and spawning phase (May-June), degenerative phase (July-August), and resting phase (September-November). And the reproductive cycle of slender catfish is similar to that of South torrent catfish.
During the mature phase of fish reproductive cycle, yolk accumulation takes place in female oocyte and spermatozoa began to appear in male seminiferous tubules. It is regulated by the hypothalamus-pituitary-gonad axis. Gonadotropin Releasing Hormone (GnRH) release occurs in the hypothalamus by stimulation of environmental factors, and GnRH induces synthesis and secretion of gonadotropin in the pituitary gland. Gonadotropin secretes estradiol in follicle of ovary, Estradiol induces synthesis secretion of vitellogenin in hepatocyte, vitellogenin accumulates in oocyte by moving blood flow to ovary. In males, 11-keto testosterone produced in testes plays an important role in spermatogenesis (Nagahama, 1987a; Nagahama, 1994).
However, even if the reproductive cycle of fish is regulated by same physiological mechanism, the environmental impact on sexual maturity in fish differ from species to species. During the mature phase, the water temperature in habitats of South torrent catfish, Black bullhead, bullhead and slender catfish was range of 9-15°C, 13°C, 18-24°C and 12-16.3°C, respectively.
Ripe and spawning phase (releasing for male) in the reproductive cycle of fish is also regulated by the hypothalamic-pituitary-gonad axis. 17α, 20β-dihyroxyprogesterone from the follicles stimulate oocyte maturation which is the division of primary oocytes with remained diplotene stage of prophase I to metaphase II. Then matured oocytes proceed the ovulation and spawning. In case of males, 11-keto testosterone and 17α, 20β-dihyroxyprogesterone released from testes induced spermiation and releasing of spermatozoa (Young et al., 1982; Nagahama, 1987b). During the ripe and spawning phase, the water temperature in habitats of for South torrent catfish, Black bullhead, bullhead and slender catfish was 21-24°C 23-28.8°C, 24-27°C and 21.5-24°C respectively. The water temperature of habitat of ripe and spawning phase also varied according to fish species. However, the temperature range of slender catfish habitat is very similar to that of South torrent catfish habitat.
Fish could be classified into six categories in respect of their spawning phase - spring spawner, spring-summer spawner, summer spawner, spring-autumn spawner, autumn spawner and winter spawner (Aida, 1991). There are three types of Siluridae fish to date: autumn spawner (Pseudobagrus fulvidraco, Liobagrus obesus, Pseudobagrus emarginatus, Leiocassis longirostris), summer spawner (South torrent catfish), and spring-summer spawner (Black bullhead) (Lim & Han, 1997; Son & Choo, 1988; Lim, 2009; Hwang, 2006; Choi, 2008). Korean slender catfish spawns from May to June, the water temperature was 21.0 °C - 24 °C and the day length was 13.59 hr. - 14.17 hr. This characteristics coincides with the spring spawner that prefers warmer and longer day length.
Fish could be classified as synchronous, group-synchronous, and asynchronous based on spawning habit. Synchronous development only have one type of developing oocyte within the ovary, and when matured it ovulates once at a specific timing. Group-synchronous development has two distinct group of oocytes in the ovaries during the spawning period. Asynchronous development has various types of oocytes within the ovary, a characteristics of multiple-spawning fish species (Shimizu et al., 1994). The slender catfish showed the synchronous oocyte development in the ovary, which is similar to South torrent catfish and Black bullhead (Choi, 2008; Hwang, 2006).
After spawning, the reproductive cycle of fish proceeds to the next stage, the degenerative phase. The water temperature during degenerative phase was 28-31°C (July and August) for South torrent catfish, 27-22°C (August and September) for Black bullhead, and 24-8°C (September to November) for bullhead. For slender catfish, it was 26.5°C in July and 28°C in August. The resting phase was September-December for South torrent catfish, October-February for Black bullhead, December-March for bullhead, and September-November for slender catfish. The growing phase was January-February for South torrent catfish, March-April for Black bullhead, April-May for bullhead, and December-February for slender catfish. The slender catfish started its’ growing phase earlier than other species.
Considering all these results, the reproductive cycle of slender catfish was very similar to South torrent catfish while quite different from Black bullhead and bullhead. The slender catfish is a Korean endemic living in small tributaries make it very vulnerable to environmental change. Currently the slender catfish has been designated as a protected wild animal in Gyeonggi Province, and it is a fish species that has been discharged for resource recovery. In addition to these efforts, we will be able to contribute to conservation of river ecosystems and preservation of endemic species in Korea through the reproductive cycle of the slender catfish in this study.
