INTRODUCTION
The olive flounder is a marine fish belonging to the flounder family, and is a representative Korean cultured-fish species that accounts for over 40% of cultured fish in Korea. Olive flounder farming has become a fast-growing food production sector, with health management of fish being crucial for sustainable industrial growth. However, competition in olive flounder farming is gradually declining due to an increase in the incidence of various diseases, deterioration of water quality due to environmental pollution, and labor and production costs. Moreover, the increase in breeding density to reduce production costs has affected the growth rate, feed intake rate, productivity, and survival rate of fish (Refstie, 1977; Rowland et al., 2006; Kim et al., 2015; Seo, 2020). In particular, mass mortality and diseases have been observed in fish due to the stress caused by an increase in breeding density (Holm et al., 1990; Björnsson, 1994; Noble & Summerfelt, 1996).
Viral hemorrhagic septicemia virus (VHSV), which causes mass mortality of farmed olive flounder, has a wide host range, including several fish species (Lee et al., 2007). VHSV is transmitted via water, urine, sperm, and ovarian fluid infected with VHSV as well as through infected live fish (Rovid, 2007). Infected fish exhibit the following symptoms: dark body color, exotometriosis, bleeding at the base of the fin, and bleeding in the internal organs and other tissues (Isshiki et al., 2001; Crane, 2006; Groocock, 2007; Duesund et al., 2010).
The immune system of aquatic animals enables them to continuously interact with microorganisms, adapt to changes in various environments, and detect and effectively defend against external pathogens (Rombout et al., 2014; Kim et al., 2020). Lymph organs in fish include the intestines, skin, and gills, and a part of the larynx also exhibits an induction function (Rombout et al., 1993). Mucosal surfaces, such as lymphoid organs, constitute the first line of defense, and mucosal health in fish is important for immunity (Rombout et al., 2014).
In the mucosa, polymeric immunoglobulin receptor (pIgR), a key component of immune defense, binds to Polymeric Immunoglobulin (pIg), enters the cell, is surrounded by endosomes, and mediates polymeric IgA (pIgA) transcytosis into the luminal surface of epithelial cells. Secretory component (SC) and IgA combine to form secretory IgA (sIgA), which is released into the mucosa (Kaetzel, 2001; Kim, 2003). The transcytosis of pIgA by pIgR blocks the attachment and invasion of potential pathogens and promotes the intracellular neutralization of viruses and bacteria, ensuring that sIgA functions as an immunological barrier (Mazanec et al., 1993; Lamm, 1997; Kaetzel, 2001; Kim, 2003). Moreover, secreted immunoglobulins (sIgs) are considered key defense molecules that bind to mucosal bacteria and neutralize antigens during the immune exclusion process (Kelly et al., 2017). SC, a soluble proteolytic portion of the extracellular domain of membrane-bound pIgR, can protect against proteolysis by pIgR-mediated epithelial cell-associated transcytosis and pIgA-associated SC (Brandtzaeg, 1985; Mostov, 1994; Mostov & Kaetzel, 1999). pIgR is a glycoprotein formed in the crude vesicle of mucosal epithelial cells and is expressed outside the base of secretory cells with domains and binds to polymeric immunoglobulins (Mostov et al., 1980; Mostov & Kaetzel, 1999). Previous studies have reported pIgR in various fish, such as Common Carp (Cyprinus carpio), dojo loach (Misgurnus anguillicaudatus), fugu (Takifugu rubripes), olive flounder (Paralichthys olivaceus), orange-spotted grouper (Epinephelus coiides), rainbow trout (Oncorhyncus mykiss) and zebrafish (Daniorerio); however, detailed investigations have not been performed (Hamuro et al., 2007; Rombout et al., 2008; Feng et al., 2009; Zhang et al., 2010; Xu et al., 2013; Yu et al., 2018). Therefore, in this study, we analyzed the expression of pIgR in olive flounder during different developmental stages and in different types of tissues. We also investigated the expression of pIgR in VHSV-infected fish according to breeding density to elucidate the role of pIgR in olive flounder immunity.
MATERIALS AND METHODS
Olive flounder were obtained from the Fish Breeding Research Center of the National Academy of Fisheries Science and bred in a 5-ton round tank (water temperature: 16±5°C), with a 15-h photoperiod and a 9-h dark cycle. The fish were fed commercial feeds of various sizes according to their growth stage. To observe the expression of pIgR at different developmental stages, whole body samples of olive flounder were collected until 50 day after hatching (DAH) from eggs, and the samples were placed in TRI-Solution™ (TS200-001, Bio Science Technology, Seongnam, Korea) and stored at −80°C until further use. Additionally, for tissue-specific expression analysis, tissue samples from the eyes, fins, gills, heart, intestine, kidney, liver, muscle, skin, spleen, and stomach were collected from 8-month-old healthy olive flounder (total length approximately 30 cm, n=3) and stored at −80°C in TRI-Solution™ until further use.
To analyze pIgR expression in artificially infected fish according to breeding density, 8-month-old healthy olive flounders (total length approximately 30 cm, n=3) were acclimatized to a 3-ton round tank and fasted the day before the experiment. The experimental group was inoculated with 100 μL of VHSV suspension (104.8 TCID50 virus/fish), and the control group was inoculated with 100 μL of phosphate-buffered saline (Kong et al., 2009). According to the standard manual for olive flounder farming and Eh (2011), breeding density was categorized as follows: high-density (coverage rate 3), standard-density (coverage rate 1), and low-density (coverage rate 0.34) (Kim et al., 2016). Tissue-sampling of the kidneys and spleen was performed every 0, 3, 6, 9, and 12 h for 10 days. The fish were anesthetized using 15 ppm MS-222 (Sigma-Aldrich, St. Louis, MO, USA), and each tissue was stored at −80°C for further experiments (Noh et al., 2017).
Total RNA was extracted from each sample using the TRI-Solution™, and cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Mannheim, Germany). qRT-PCR was performed according to the method described by Kim et al. (2021), and the 18S rRNA gene was used as the internal standard control. mRNA expression was quantified using the 2−ΔΔCt method (Pfaffl, 2001). The primers for the experiment were designed using the Primer3 program and the NCBI database (Rozen & Skaletsky, 2000). The nucleotide sequence of the primers is as follows: pIgR primer (Gene bank, HM536144.1), forward: 5′-AGCCTCAGTATGCCAGCAAT-3′; reverse: 5′-GCACCTGTACCACCCAGAGT-3′ and 18S rRNA primer (Gene bank, EF126037.1), forward: 5′-ATGGCCGTTCTTAGTTGGTG-3′; reverse: 5′-CACACGTGATCCAGTCAGT-3′. The results of this experiment were statistically analyzed using R 3.0.1 software (Okorie et al., 2013). The Shapiro–Wilk test was performed to test the normality of the data, and it was found that the normality test was not violated (p<0.05). A one-way analysis of variance (ANOVA) was performed to verify the statistical significance of the difference between the means of the groups. When significance was detected (p<0.05), a multiple range test was performed using Tukey’s honestly significant difference (HSD). All data are expressed as mean±standard error, and p-values indicate the statistical significance for all analyses. This article has been approved by IACUC by the Animal Experimental Ethics Committee (2020-NIFS-IACUC-17).
RESULTS
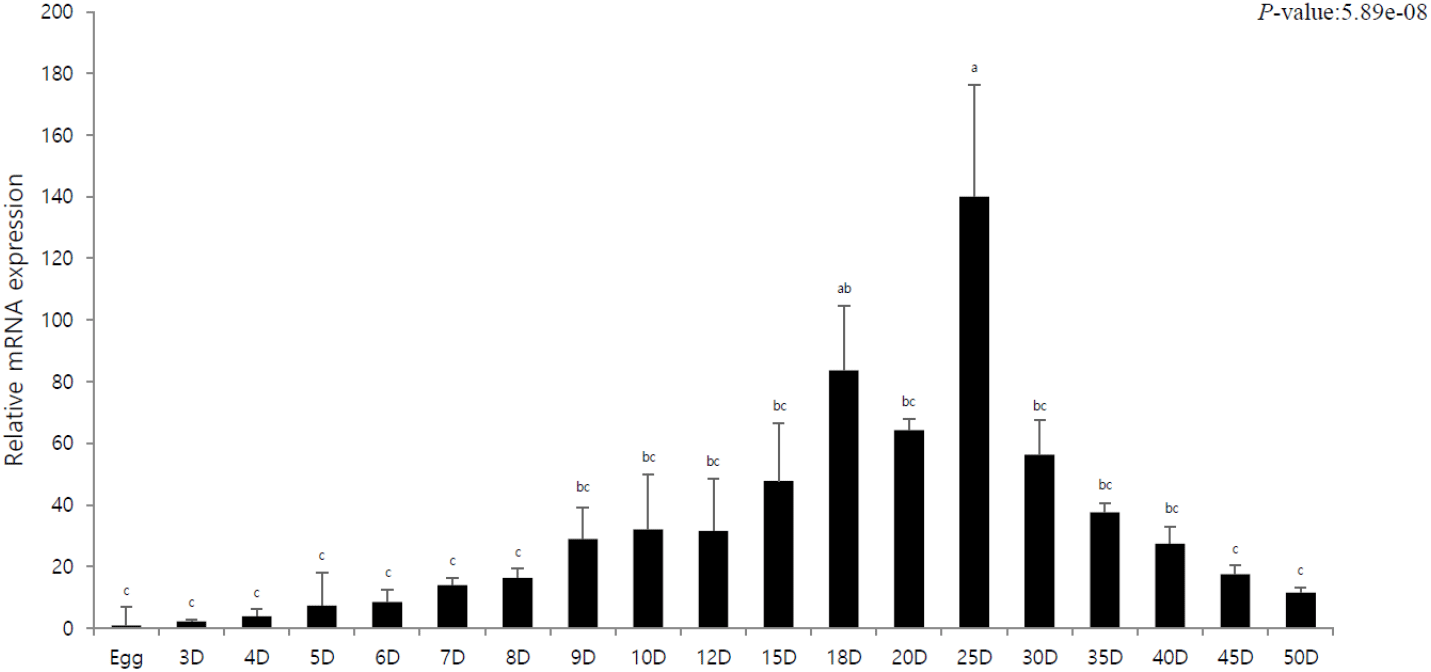
The purpose of this experiment was to elucidate the innate immune response of olive flounder by investigating pIgR expression according to the developmental stage. The mRNA expression of pIgR was quantitatively analyzed using pIgR-specific primers at different developmental stages, and the expression was compared to that of egg (=1). We observed that pIgR expression gradually increased from 0 DAH, and exceeded 30-fold after 10. The highest expression was observed on 25 DAH, after which the expression rapidly decreased; moreover, between 30 DAH and 50 DAH, a gradual decrease in the expression was observed.
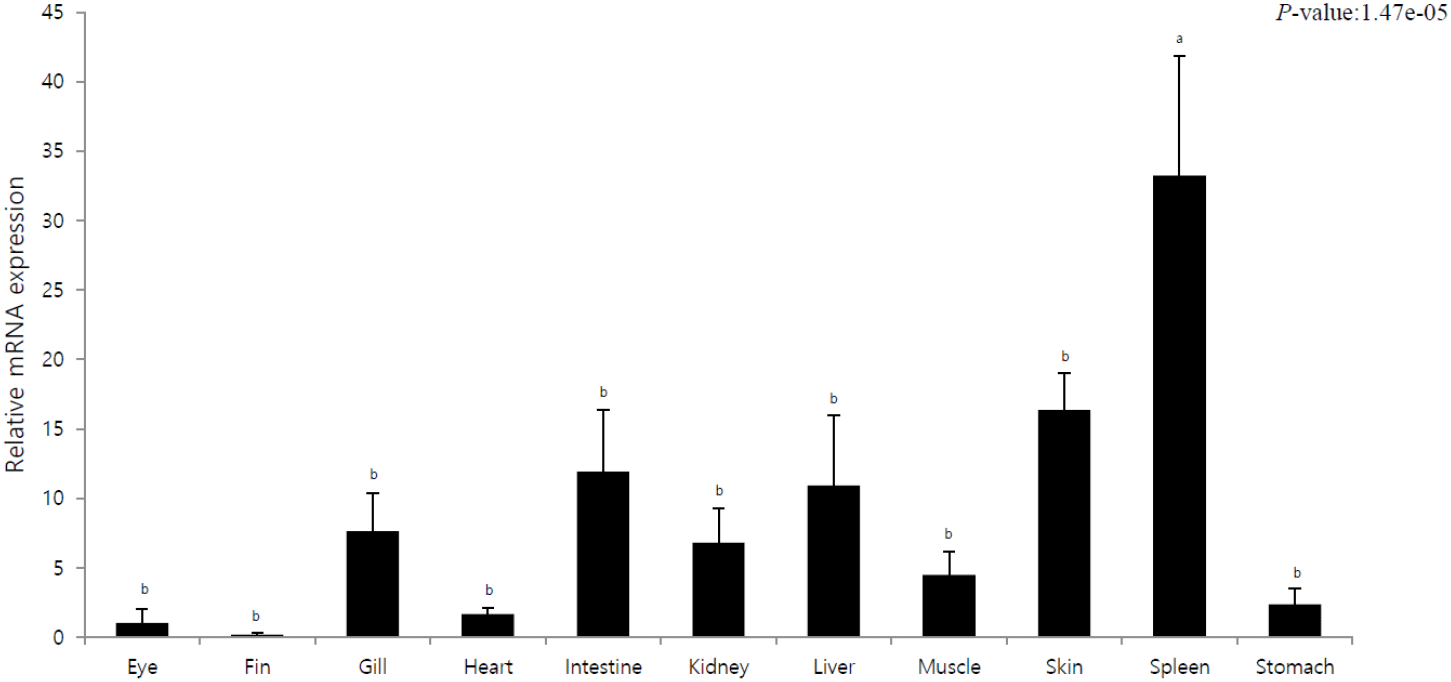
The purpose of this experiment was to elucidate tissue-specific immune responses of olive flounder by investigating pIgR expression in different tissues. Tissue-specific mRNA expression was quantitatively analyzed using pIgR-specific primers, and the expression was compared to that in the eyes (=1). pIgR expression was the lowest in the fins and eyes and the highest in the spleen. Moreover, the liver, intestine, and skin exhibited 10-fold higher expression than the eyes. In other tissues (gills, heart, kidney, muscle, and stomach), the expression was 2–8 times higher than that in the eyes.
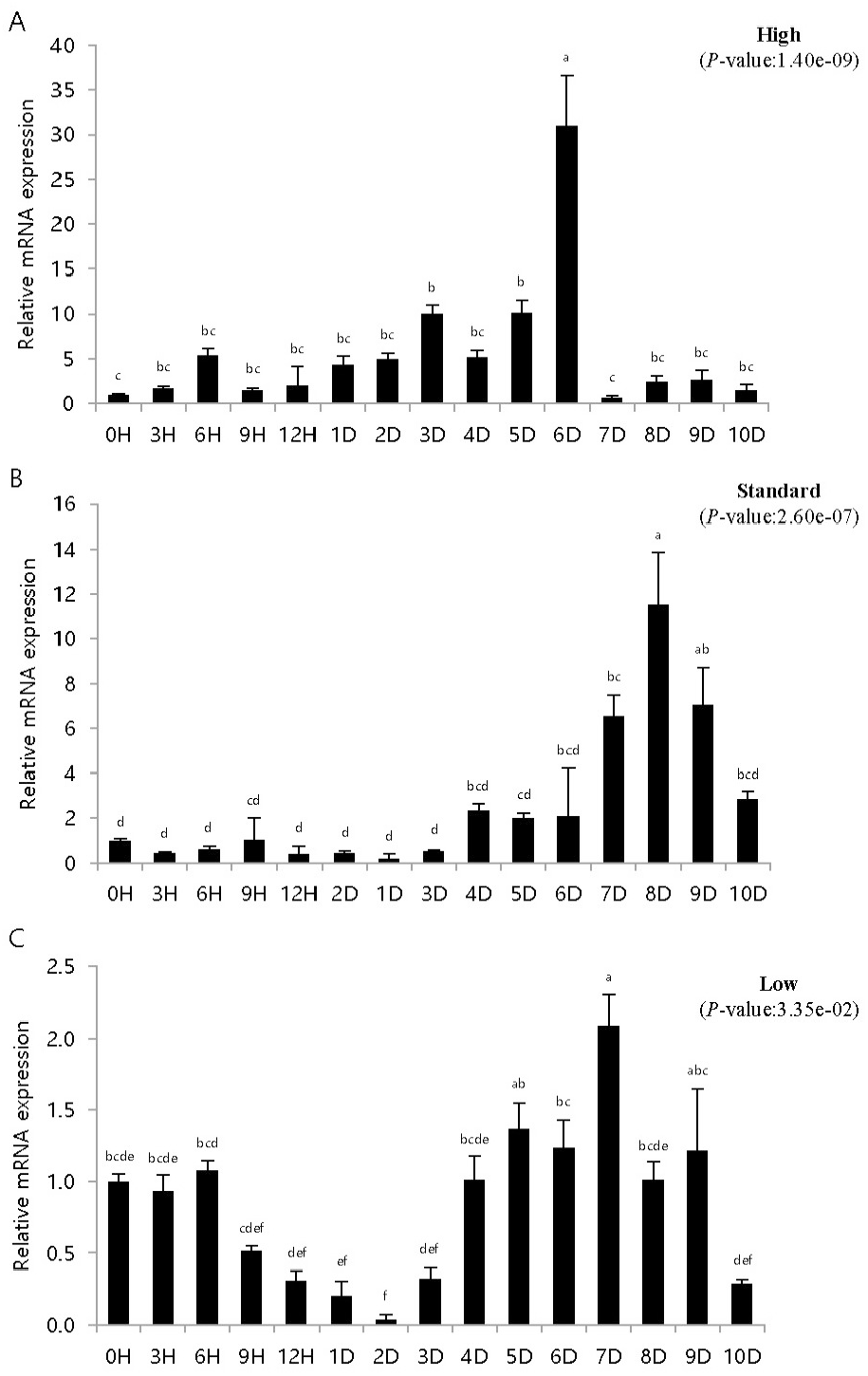
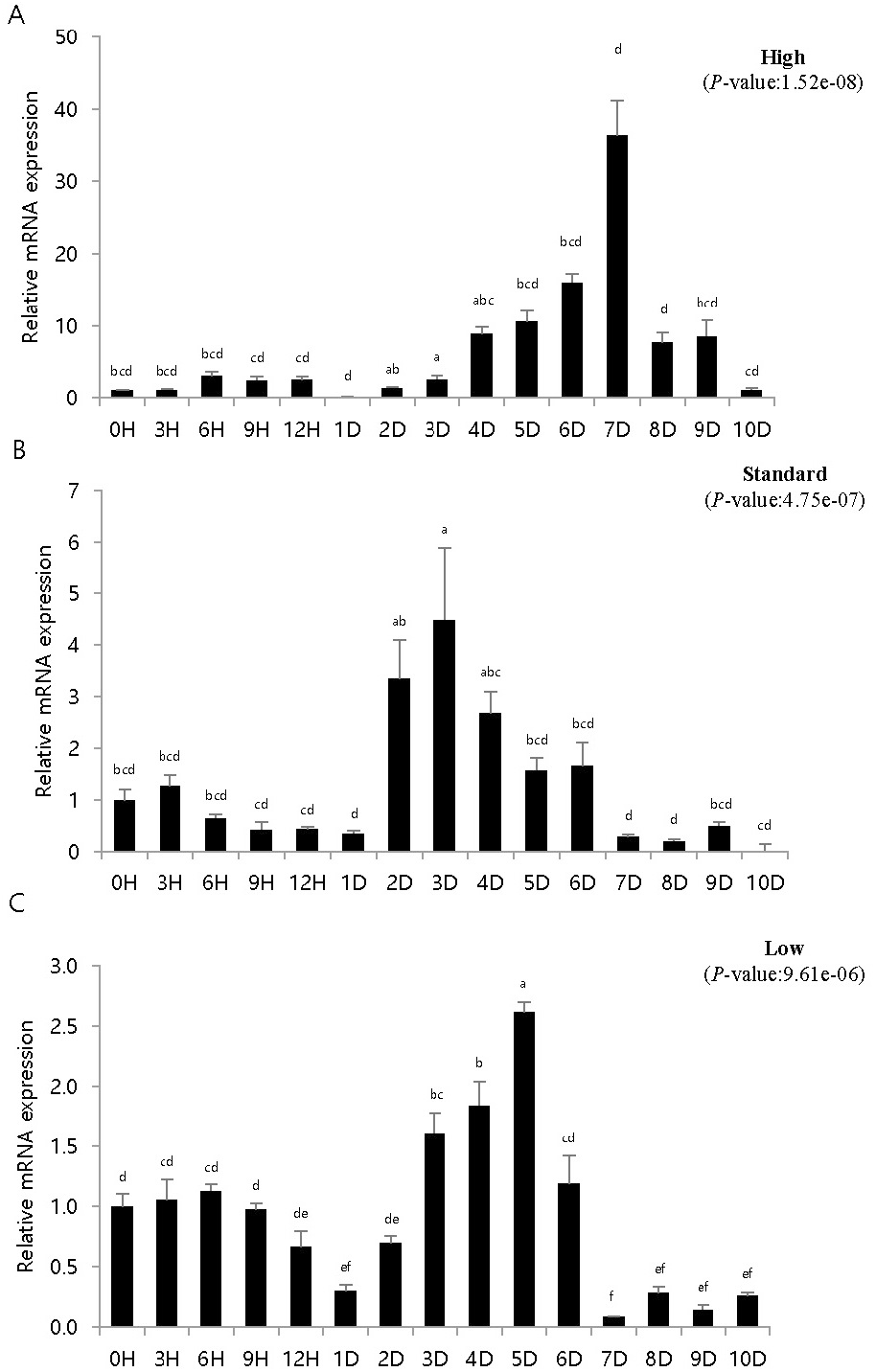
To study the immune response of fish artificially infected with VHSV according to breeding density, pIgR expression was investigated in artificially infected fish bred at different densities. pIgR mRNA expression was quantitatively analyzed by density and time using pIgR-specific primers, and the expression was compared to that at 0 h (=1). The experiments were conducted using kidney and spleen tissues, which are highly correlated with immune response.
Control inoculated with phosphate buffered saline showed similar results to those at 0 h in density and time-dependent expression values (data not shown). The height in the high-density breeding tank initially increased and then decreased, but on day 6, pIgR expression was over 30 times higher than that at 0 h and then rapidly decreased. The height in the standard density breeding tank did not exhibit any significant change until day 3; however, pIgR expression more than doubled on day 4, exhibiting the highest expression on day 8 and then decreased rapidly. The height in the low-density breeding tank decreased from 0 h until day 2 of inoculation, then gradually increased on day 3; pIgR expression was double on day 7 and decreased thereafter.
In the spleen samples from the high-density breeding tank, pIgR expression increased over nine times on day 4 compared to that at 0 h and exhibited the highest expression on day 7. Subsequently, the expression decreased sharply, and on day 10, the expression was the same as that on day 1. The expression in the spleen samples from the standard-density breeding tank did not exhibit substantial change until day 1, but on day 2, pIgR expression increased over three times compared to that at 0 h of inoculation; the highest expression was observed on day 3, after which it gradually decreased. After day 7, the expression was lower than that at 0 h. The expression in the spleen samples from the low-density breeding tank remained the same or decreased until day 2, but increased from day 3, exhibiting the highest expression on day 5; after day 7, the expression was lower than that at 0 h.
DISCUSSION
Fish are susceptible to diseases caused by highly infectious pathogens and in poor rearing environments. High-density intensive fish farming methods in Korea have led to the deterioration of farm environments and increased the incidence of various diseases by inducing stress (Lee et al., 2002). Fish live in an aquatic environment, and their mucosal surface is exposed. Thus, from a young age, the surface acts as an invasion route for numerous microorganisms (Kim et al., 2020). Therefore, mucosal health is more important in fish than in other animals (Rombout et al., 2014). pIgR is an essential component of the immune system that mediates transdermal transport of slgs to protect organisms from environmental pathogens and is the most important mucosal effector (Xu et al., 2013; Sheng et al., 2022). In fish, plgR is a single transmembrane protein that consists of extracellular, transmembrane, and intracellular domains (Kaetzel et al., 1997; Hamburger et al., 2004). Fish plgR has two immunoglobulin-like domains, and olive flounder plgR has a unique structure (Wieland et al., 2004; Hamuro et al., 2007; Rombout et al., 2008; Feng et al., 2009; Tadiso et al., 2011; Xu et al., 2013).
In this study, pIgR was gradually expressed after hatching and exhibited a rapid increase in expression as the digestive tract entered the period of complete differentiation. We observed the highest expression at the stage where the lymphocytes were formed, i.e., the immune system developed (Fig. 1). The immune system in fish includes the liver, spleen, and kidneys; these organs along with the skin and gills are considered mucosal lymphoid organs (Goldblum, 1990). In case of marine fish, the order of development of the major lymphoid organs is kidneys, spleen, and thymus, and the timing of complete development of these organs determines the stage with full immune capacity, not just the morphological meaning (Nakanishi, 1986; Jósefsson & Tatner, 1993; Pulsford et al., 1994; Padrós & Crespo, 1996; Schroder et al., 1998; Zapata et al., 2006). The results of this study confirmed that pIgR expression gradually decreased after the complete development of the immune system but was maintained (Fig. 1). This shows that pIgR, as a key component of immune defense, plays an important role in the immune response of olive flounder after hatching. Moreover, in the present study, pIgR was expressed in all tissues of olive flounder, with the highest expression observed in the spleen and high expression in the skin, liver, and intestines (Fig. 2). These results were similar to those previously reported for olive flounder and other fish, and the high expression in immune tissues suggests that plgR is an important effector of the immune system (Rombout et al., 2008; Xu et al., 2013; Yu et al., 2018; Sheng et al., 2022).
Olive flounder is an economically important fish species in Asian countries, such as Japan, Korea, and China (Park et al., 2015). However, many infectious diseases have been reported in olive flounder depending on different breeding conditions, such as water temperature, density, and quality, and have caused heavy economic losses (Kim et al., 2009; Kim et al., 2010). VHSV is a hemorrhagic septicemia virus, and the incidence of its infection is affected by culture density, water temperature, nutritional status, and water quality (Kim et al., 2021). In particular, high breeding density creates a conducive environment for the spread of diseases and causes mass mortality, whereas low density inhibits growth by reducing feed intake (Holm et al., 1990; Brock, 1992; Oh et al., 2013). Stress in fish is known to be closely related to immunity, and stress in fish due to high density increases both plasma cortisol level and reactive oxygen species formation, which induces oxidative stress (Vijayan et al., 1990; Braun et al., 2010; Psaltopoulou et al., 2010; Yarahmadi et al., 2015). Kim et al. (2016) set the coverage rate as the standard for calculating the stocking density. The kidneys and spleen exhibited high and low expression at high and low densities, respectively (Figs. 3, 4). Moreover, at low densities, pIgR expression decreased and increased after 1–2 days of the experiment (Figs. 3, 4). These results differ from those reported in previous studies that investigated IRF3 expression in the diseased state at different breeding densities (Kim et al., 2021). Moreover, diseased flatfish may exhibit different immune responses depending on the density and genes. The results of this study provide important basic data for determining breeding density in fish farming and for immunogenetic studies based on disease and breeding density.