INTRODUCTION
The maintenance of adult organs depends on the intricate regulation of stem cell self-renewal and differentiation. Stem cell behavior is governed by its local microenvironment, termed the stem cell niche (Morrison & Spradling, 2008), which plays a pivotal role in adult organ maintenance. Niche signaling to stem cells is relatively well studied, but how a niche is established during development remains poorly understood.
In the Drosophila male gonad, a single niche comprised of hub cells is located at the apical tip of the testis, wherein it contacts and regulates the behaviors of adjacent stem cells of two types, germline stem cells (GSCs) and cyst stem cells (CySCs) (Gönczy & DiNardo, 1996; Kiger et al., 2001; Greenspan et al., 2015). GSCs self-renew to produce a goniablast, which undergoes four mitotic cell divisions to produce 16 germline cells, while CySCs self-renew to produce somatic cyst cells that enwrap the germline cells (Zoller & Schulz, 2012). The niche secretes signaling molecules, including unpaired, decapentaplegic, glass bottom boat and hedgehog that govern stem cell behaviors and stemness (Greenspan et al., 2015; Eslahi et al., 2024). However, the mechanism by which this single apical niche is formed is largely unknown.
The apical location of the gonad niche is established during embryogenesis (Le Bras & Van Doren, 2006; Dinardo et al., 2011; Zamfirescu et al., 2022). Niche cells are specified from somatic gonadal precursor cells of mesodermal origin, and in mid-embryos are initially dispersed throughout the spherical gonad (Boyle & DiNardo, 1995; Le Bras & Van Doren, 2006; Kitadate & Kobayashi, 2010; Okegbe & DiNardo, 2011). Subsequently, posteriorly located niche cells migrate anteriorly to assemble with the anteriorly located niche cells, thereby forming a single niche at the gonad anterior (Anllo et al., 2019; Anllo & DiNardo, 2022). Defects in the signaling directing this migration result in the dispersed formation of an extra niche (Anllo & DiNardo, 2022); however, the molecular mechanisms of anterior migration and niche assembly are still poorly understood.
Lin28 is an evolutionarily conserved RNA-binding protein and also a regulator of let-7 microRNA genesis (Krsnik et al., 2022). In the adult Drosophila testis, Lin28 is exclusively expressed in niche cells, wherein it has been demonstrated to be required for the maintenance of the number of niche cells and niche architecture (Sreejith et al., 2019). Here, we find that Lin28 is required in niche cells for the formation of a single niche in the embryonic gonad.
MATERIALS AND METHODS
Immunostaining was done as previously reported (Fidler et al., 2014). Briefly, third instar male larvae were dissected in Schneider’s media. The posterior region of the dissected larvae, containing the testis, was fixed in 4% formaldehyde in phosphate-buffered saline for 20 minutes. The larvae were then incubated in primary antibodies overnight at 4℃. The next day, secondary antibody treatment was performed, and then the testis was carefully detached from the posterior region using tungsten needles and mounted with an antifade reagent. The following primary antibodies were used at the indicated concentrations: rat anti-VASA 1:400 (Developmental Studies Hybridoma Bank, DSHB) and guinea pig anti-Traffic Jam 1:10,000 (Dorothea Godt, Toronto, ON, Canada). The corresponding secondary antibodies were used at 1:500 concentration (Alexa Series, Invitrogen, Carlsbad, CA, USA).
Flies were reared in standard cornmeal/agar medium at 25℃ with 50% humidity and a 12 h light/dark cycle. W1118 (used as wild-type) and lin-28EP915 were obtained from the Bloomington Drosophila Stock Center. esg-GAL4 was a kind gift from S. Dinardo. UAS-lin28 was generated in the lab (Sreejith et al., 2019).
RESULTS AND DISCUSSION
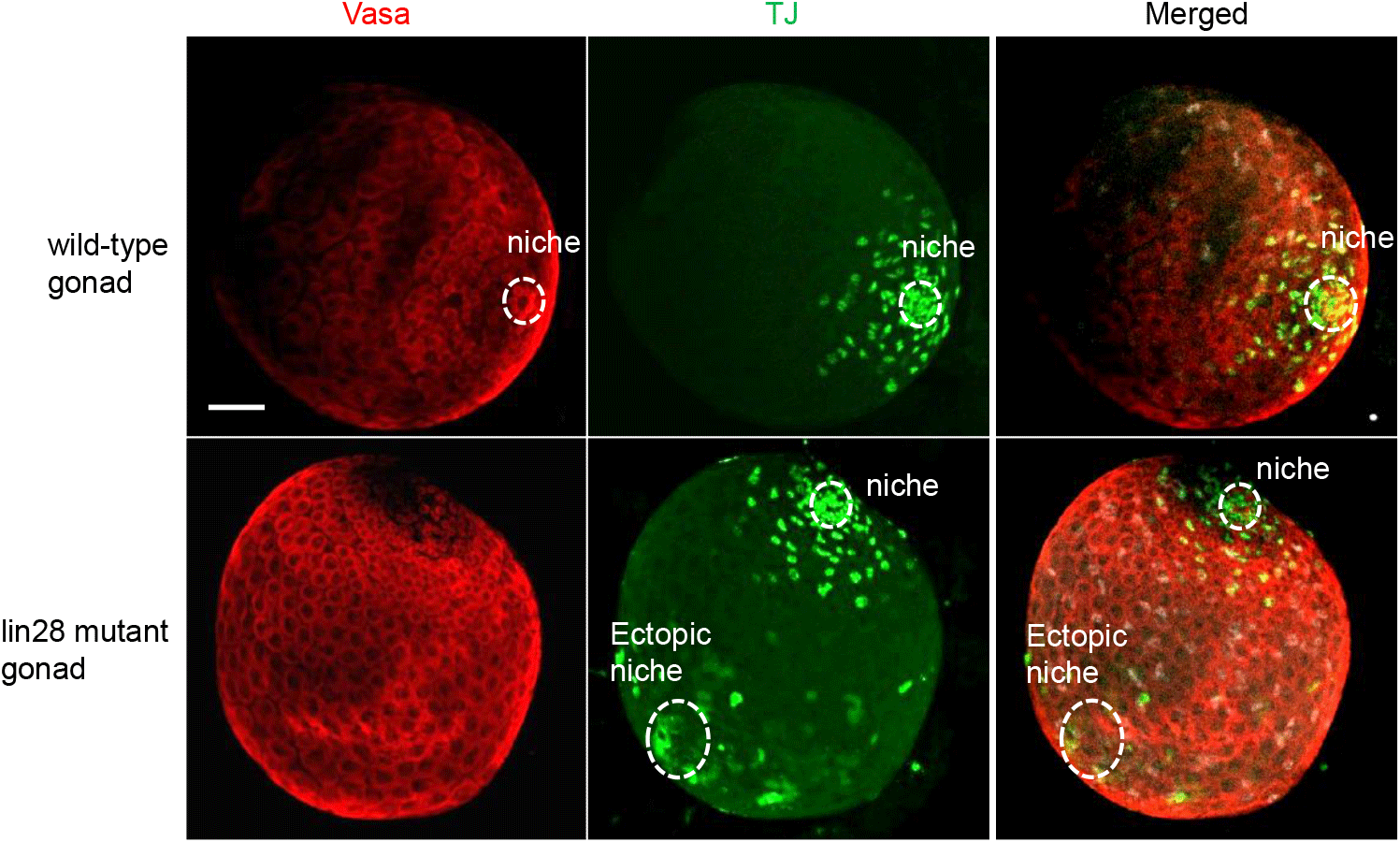
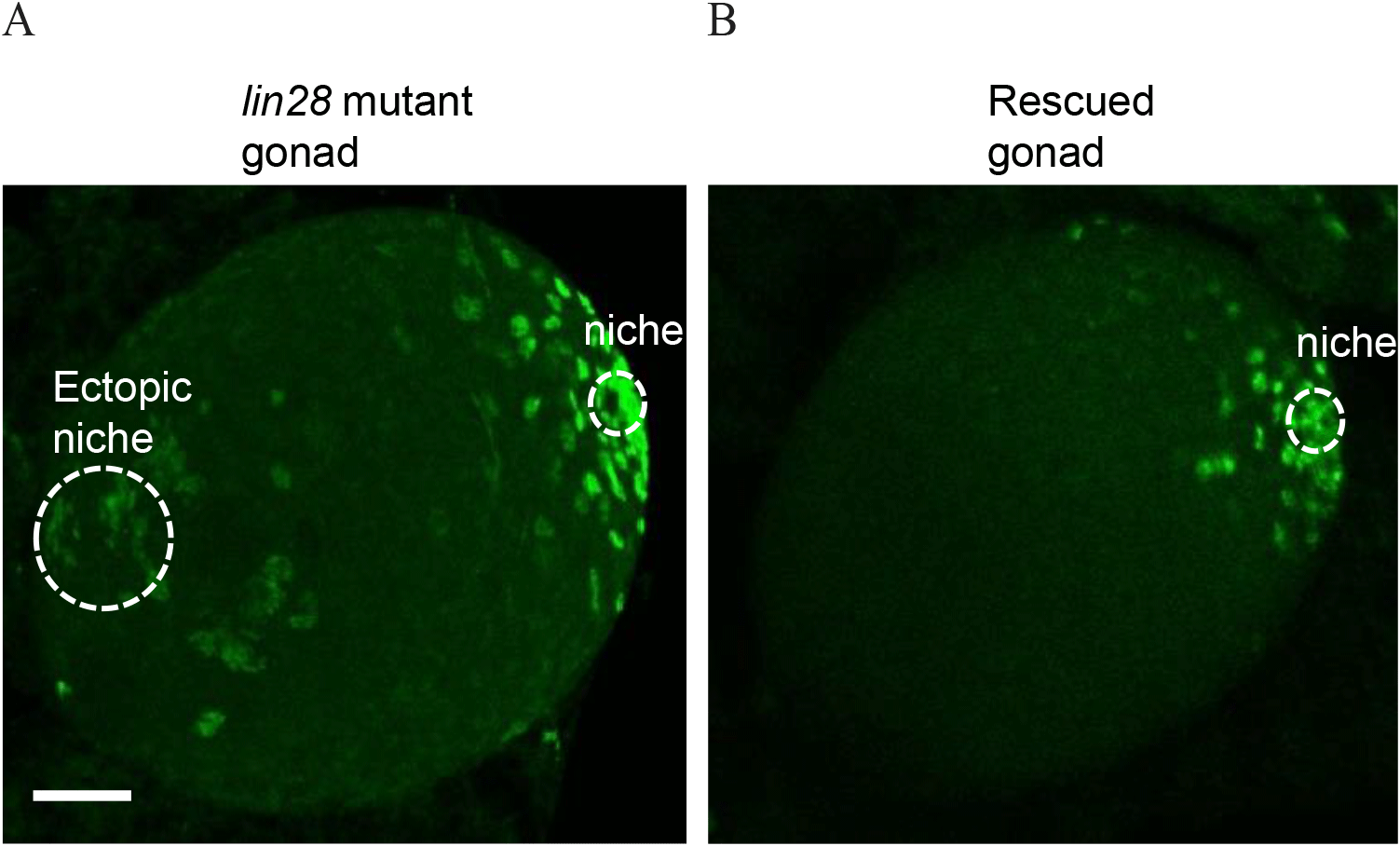
We marked niche cells using antibodies against Traffic jam (Tj), a Maf transcription factor (Li et al., 2003; Sinden et al., 2012; Fairchild et al., 2016), which is expressed in niche cells and germline cells using Vasa (Sinden et al., 2012; Sreejith et al., 2019). Immunohistochemistry of wild-type larval male gonads showed a single niche, while lin28 mutant gonads (lin28EP915) (Sreejith et al., 2019) demonstrated dispersed, ectopic niches (Fig. 1). These findings suggest that Lin28 is required for the formation of a single niche in the gonads. To test this potential requirement, we sought to express Lin28 specifically in niche cells within the lin28 mutant male gonad. We employed a Gal4/UAS binary expression system in which the UAS-downstream gene is expressed in specific cells under the Gal4 driver (Brand & Perrimon, 1993). To express Gal4 in the niche cells, we used the esg-Gal driver (Voog et al., 2008, 2014; Sênos Demarco et al., 2022). The UAS-lin28 line was previously generated in our lab (Sreejith et al., 2022). With this system, we generated a rescue line that harbors esg-Gal4 and UAS-lin28 in the lin28 mutant background (esg-Gal4, UAS-lin28; lin28EP915/ lin28EP915), and therefore expresses lin28 in niche cells within a lin28 mutant male gonad. Immunohistochemistry demonstrated that individuals of this rescue line exhibit a single niche, indicating that Lin28 is required in niche cells to form a single niche in the gonads (Fig. 2).
The Drosophila testis contains a single niche at the apical tip of the gonad (Kiger et al., 2001). How this single niche is formed is largely unknown, despite such mechanisms being of prime interest in the stem cell field. Here we show that Lin28 is required in gonad niche cells for this process of single niche formation. Firstly, we show that the formation of a single niche is aberrantly disrupted in lin28 mutant gonads. Secondly, specific expression of lin28 in niche cells in the lin28 mutant background restores the single niche. These data highlight a requirement of Lin28 in niche cells for the formation of a single niche.
In the development of this singular niche, niche cells are initially dispersed throughout the gonad (Le Bras & Van Doren, 2006; DeFalco et al., 2008; Anllo et al., 2019; Anllo & DiNardo, 2022). Those located posteriorly then migrate anteriorly and assemble with their anteriorly located counterparts to produce the single niche (Anllo et al., 2019; Anllo & DiNardo, 2022). Defects in this anterior migration process are known to result in a dispersed, ectopic niche in the gonads (Anllo & DiNardo, 2022). The observation of similar phenotypes in lin28 mutant gonads supports the proposition that Lin28 might play a role in the anterior migration of niche cells (Fig. 3).
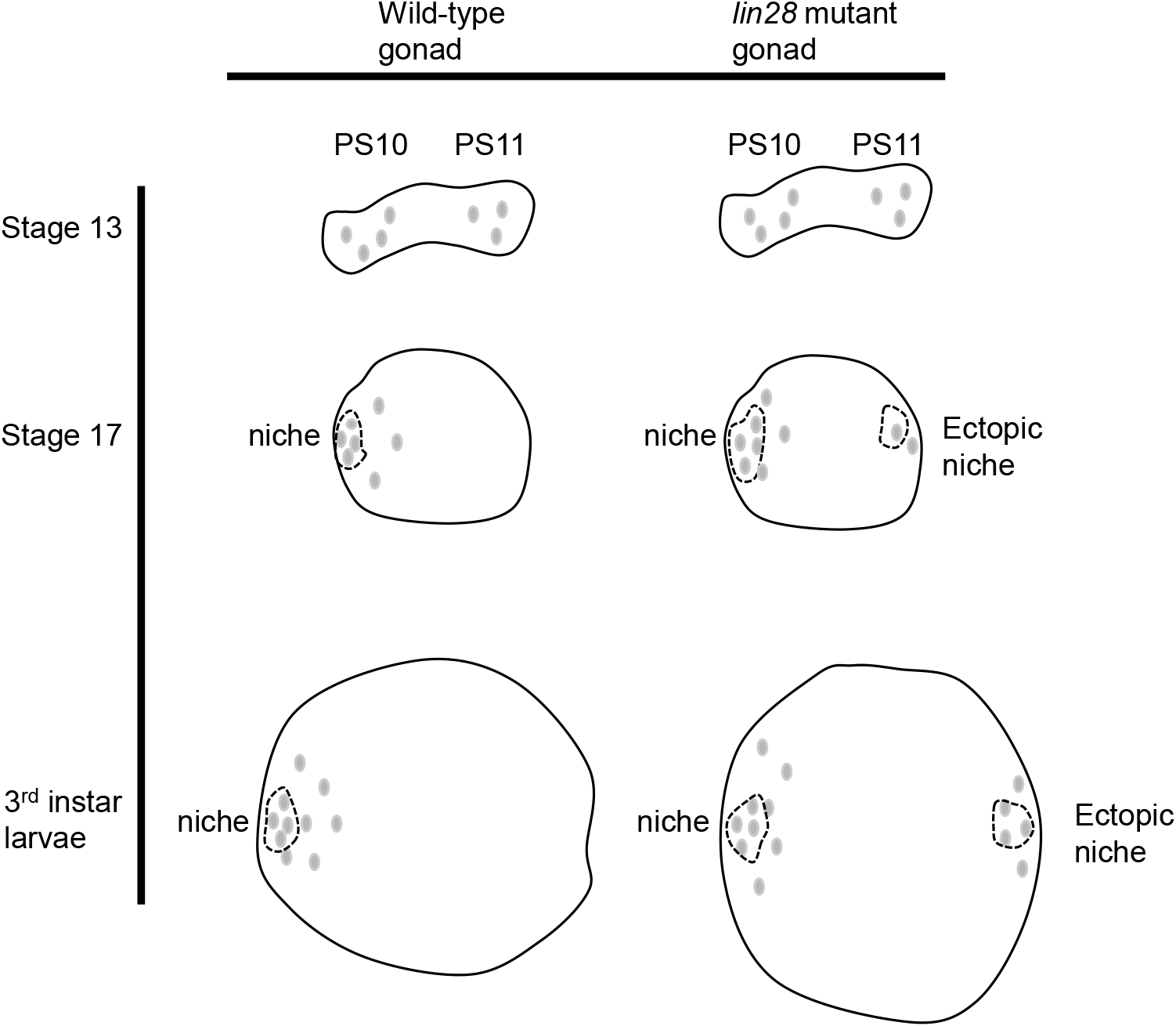
This raises the question: What function does Lin28 perform in niche cells? Studies have shown that Lin28 mediates the stability of niche-specific mRNAs in niche cells (Sreejith et al., 2019; To et al., 2021). Thus, Lin28 might stabilize mRNAs that are themselves involved in the anterior migration of niche cells. Candidate targets of Lin28 include the transcription factor islet, which was recently shown to be expressed in niche cells and required for anterior niche cell migration (Anllo & DiNardo, 2022), and also regulators and components of the actin cytoskeleton, which mediate cell migration in diverse cellular contexts. Alternatively, Lin28 is also known to regulate the genesis of let7 microRNAs, which might play a role in the anterior migration. Future research is required to reveal the detailed mechanism by which Lin28 regulates the development of a single niche in the Drosophila male gonad.
