INTRODUCTION
The giant freshwater prawn (Macrobrachium rosenbergii) is a major freshwater aquaculture species widely farmed in tropical and subtropical regions (New, 2005). According to the Food and Agriculture Organization of the United Nations (FAO), global aquaculture production of M. rosenbergii exceeded 312,000 tons in 2020 (FAO, 2024), highlighting its substantial economic importance in the aquaculture industry. Consumers generally prefer organisms with vivid and intense coloration, which in crustaceans is produced through the accumulation of carotenoids (Latscha, 1989). However, crustaceans are unable to synthesize carotenoids endogenously and therefore depend entirely on dietary sources (Goodwin, 1952). As a result, insufficient carotenoid intake can lead to poor coloration, reduced marketability, and increased physiological stress (Wade et al., 2015; Long et al., 2017).
Carotenoids are terpenoid pigments widely distributed in nature and are synthesized by plants, algae, and certain microorganisms. They typically appear in shades of red, orange, and yellow (Lu & Li, 2008). Structurally, carotenoids are classified into carotenes and xanthophylls. Carotenes, such as β-carotene and lycopene, lack oxygen in their structure, whereas xanthophylls include astaxanthin, lutein, and zeaxanthin, contain oxygen (Jackson et al., 2008). Carotenoids contribute to growth, immunity, and survival through various physiological activities that extend beyond their role as pigments (Choi & Kim, 2022). In particular, they possess strong antioxidant properties, protecting cellular structures from reactive oxygen species (ROS) and enhancing immune cell function (Jackson et al., 2008). In crustaceans, dietary carotenoids accumulate primarily in the hepatopancreas, ovaries, epidermis, and carapace, where they generate diverse coloration patterns that directly influence the intensity of body color (Li et al., 2020).
Unlike vertebrates, the invertebrate M. rosenbergii lacks an adaptive immune system and depends on innate immune responses to defend against external pathogens (Tassanakajon et al., 2015; Zhao et al., 2022). Antimicrobial peptides (AMPs) are components of the humoral immune system and play essential roles in protecting the organism from invading pathogens. Crustin is one of the numerous AMPs identified in crustaceans. The first crustin was reported in the Carcinus maenas and was named carcinin (Schnapp et al., 1996). Crustin is primarily expressed in circulating hemocytes within the hemolymph system (Tassanakajon et al., 2015) and has been shown to exhibit antimicrobial activity against Gram-positive bacteria (Matos & Rosa, 2022). Upon pathogen infection, crustin expression increases and contributes to pathogen elimination through enhanced phagocytosis and the regulation of immune-related genes (Zhao et al., 2022).
Furthermore, the health status of shrimp is closely associated with the maintenance of oxidative homeostasis within their bodies. Stress factors common in aquaculture environments, including high-density farming, fluctuations in water quality, and pathogen invasion, can trigger excessive accumulation of ROS. This accumulation leads to lipid peroxidation of cell membranes, protein denaturation, and DNA damage, which severely compromise cellular function (Chew & Park, 2004; Nath & Haldar, 2020). Superoxide dismutase (SOD), one of the primary antioxidant enzymes responsible for ROS removal, converts the superoxide radical (O₂⁻), a type of ROS, into the relatively less harmful hydrogen peroxide (H₂O₂) and oxygen (O₂) (Chew & Park, 2004). Therefore, the function of the antioxidant defense system, including SOD activity, is considered a key factor in maintaining shrimp health and enhancing their immune capacity (Nath & Haldar, 2020).
Several studies have reported that supplementing carotenoids in feed has beneficial effects not only on improving the body color of crustaceans but also on enhancing antioxidant activity, immune function, and survival rates. Research using Forsythia koreana leaves in the diet of M. rosenbergii (Kim et al., 2023) and mangrove leaves for Penaeus monodon (Alam et al., 2022) has demonstrated that terrestrial plants can improve body coloration. However, terrestrial plants collected from nature environments pose risks of contamination and pathogen exposure, and their slow growth rates make large-scale production within a short period challenging. Therefore, there is a need to identify stable and efficient plant-based sources of carotenoids that can address these limitations.
The microalgae Haematococcus lacustris is gaining attention as an alternative capable of overcoming these limitations. H. lacustris is a single-celled freshwater microalgae that contains large amounts of astaxanthin, a carotenoid pigment (Boussiba, 2000). As a survival strategy under environmental stress conditions such as high light intensity, nutrient deficiency, and salinity changes, it biosynthesizes astaxanthin in large quantities and can accumulate it up to 3%–5% of its dry weight (Khoo et al., 2019; Bas et al., 2021), which makes it highly valuable for industrial use. It can also be mass-cultured under controlled conditions and has a doubling time of approximately 5 days (Sipaúba-Tavares et al., 2023), allowing stable production within a short period. Astaxanthin is regarded as a particularly effective supplement because of its strong antioxidant capacity and its ability to enhance pigmentation (Chien & Jeng, 1992; Long et al., 2017). It is known to prevent cellular damage through its potent suppression of oxidative stress and to contribute to maintaining the physiological adaptability of crustaceans (Wu et al., 2017).
Therefore, in this study, a 60-day feeding trial was conducted to evaluate the effects of H. lacustris supplemented diet on the growth, body color enhancement, expressions of SOD and crustin genes, and also on the histological changes of the hepatopancreas in M. rosenbergii post-larvae.
MATERIALS AND METHODS
Experimental diet was prepared by adding freeze-dried H. lacustris powder (astaxanthin content: 3% w/w) to commercial feed (Daeha Plus Start, Woosung Feed, Daejeon, Korea) (Table 1). Three experimental diets were formulated based on the astaxanthin content: control (CON, 0 mg/kg diet), low concentration (LC, 50 mg/kg diet), and high concentration (HC, 200 mg/kg diet). The dietary astaxanthin levels were determined based on previous studies conducted in Lipopenaeus vannamei (Niu et al., 2009) and Marsupenaeus japonicus (Chien & Shiau, 2005). To achieve the experimental astaxanthin levels, 1.66 g/kg and 6.64 g/kg of H. lacustris powder was added to LC and HC, respectively (Table 2).
M. rosenbergii larvae for the experiment were produced by mating adult male and female prawns grown in fish rearing facility at Sunmoon University. The larvae were accommodated into nine tanks (180 L, each) at a rate of 120 individuals per tank. PVC corrugated sheets (Plavenia) were placed over the tanks to prevent the prawn from escaping. Sizes of prawn larvae were 19.8±0.8 mg (weight) and 1.08±0.20 cm (total length) at the beginning of the experiment. The experimental diets were provided twice a day at 9:00 AM and 6:00 PM. Fifty percent of the rearing water was replaced daily, and the water temperature was maintained at 28±1°C throughout the 60-day rearing experiment.
Before placing them in the experimental tanks, 40 prawns were randomly collected. Among these, 30 were weighed and measured for total length, and their hepatopancreas and tail muscle tissues were collected for genetic analysis and stored at −80°C. The remaining 10 were photographed for body color analysis.
On days 30 and 60 of rearing, five prawns were randomly collected from each tank to measure growth, and their weight and total length were recorded. After measurement, the hepatopancreas and tail muscle were collected and stored at −80°C for gene expression analysis. Survival rate was calculated by counting the number of prawns remaining in each tank at the end of the experiment.
To analyze body color changes at 30 and 60 days of cultivation, ten prawns were randomly collected from each tank and photographed. Photography was conducted from the side after placing each prawn in a transparent acrylic tank. The camera settings were fixed at ISO 50, shutter speed 1/500 s, and white balance 3,500 K. Lighting (Panasonic LED Smart Stand, Panasonic, Osaka, Japan) was used inside a darkroom to maintain consistent lighting conditions. The captured images were quantitatively analyzed using Adobe Photoshop software by measuring the R (red), G (green), and B (blue) values in the striped area at the base of the eye stalk, where coloration first appears.
At the end of the experiment, ten prawns were randomly selected from each tank to observe body color changes after heating and stored at −20°C. Subsequently, they were then heated in boiling water at 100°C for 3 minutes, after which the body color was observed.
Gene expression was analyzed using hepatopancreas and tail muscle tissues stored at −80°C. Total RNA was extracted using Trizol™ Reagent (Ambion, Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s protocol. The extracted RNA was quantified using a spectrophotometer (NanoDrop 2000, Thermo Scientific, Waltham, MA, USA). cDNA was synthesized using TOPscript™ RT DryMIX (Enzynomics, Daejeon, Korea), and the remaining RNA was stored at −80°C.
The synthesized cDNA was mixed with 10 μL Topreal™ qPCR 2X PreMIX SYBR Green (Enzynomics, Daejeon, Korea), 1 μL of forward primer, 1 μL of reverse primer, diethylpyrocarbonate (DEPC) (Intron, Seongnam, Korea) 3 μL, and 5 μL of 50-fold diluted cDNA, resulting in a total volume of 20 μL. The primer sequences used in this are listed in Table 3.
Quantitative real-time polymerase chain reaction (qRT-PCR) conditions were with the following conditions. The reaction began with an initial denaturation (95°C, 15 min), denaturation (95°C, 10 sec), annealing (60°C, 15 sec), elongation (72°C, 20 sec). Reactions were performed for a total of 40 cycles using the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). After the reaction, the melting curve was analyzed by relative quantification using the β-actin gene as a control gene.
The hepatopancreas of each individual was collected for histological analysis after body color photography. The tissue was fixed in a 10% neutral formalin solution for 24 hours, then dehydrated, cleared, and embedded in paraffin. To prevent tearing during sectioning, the paraffin block was immersed in a mixture of 60% alcohol and glycerol in a 1 to 9 ratio for 24 hours to soften the tissue. Sections with a thickness of 5 μm were prepared using a microtome. After being dried for 24 hours, the sections were stained with hematoxylin and eosin and examined under an optical microscope to assess tissue condition.
All data are expressed as the mean±SEM. Survival rate, growth performance, body color, and gene expression levels were analyzed using one-way ANOVA (GraphPad Software, San Diego, CA, USA). When significant differences between experimental groups were analyzed using Duncan’s test and SPSS Statistics 20 (p<0.05).
RESULTS
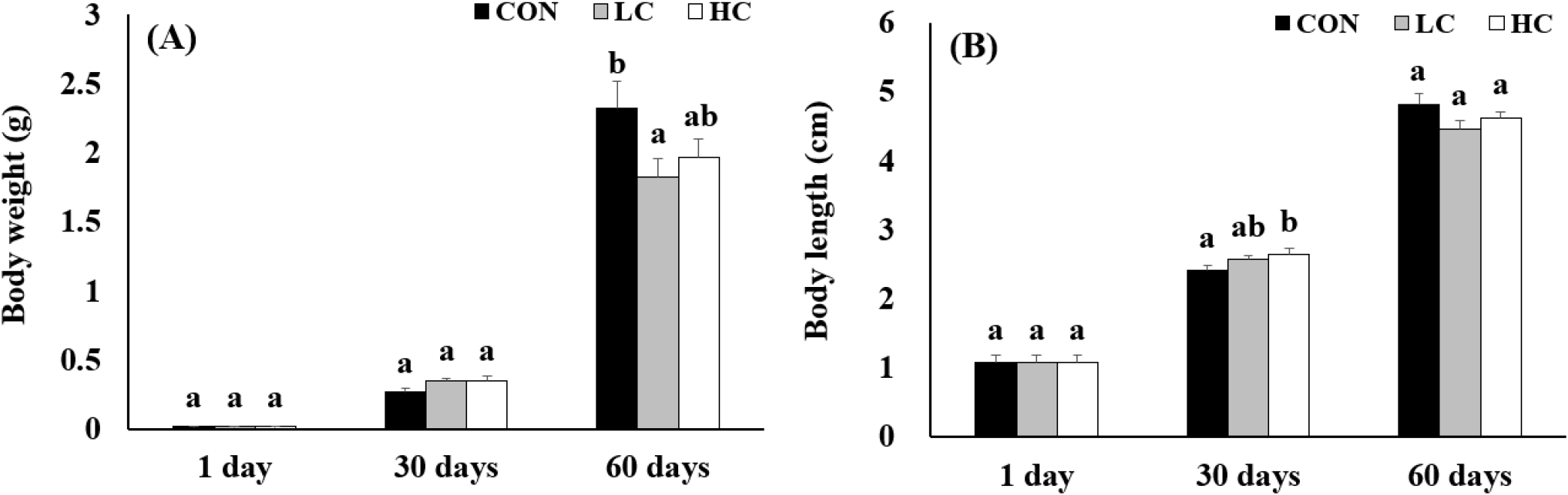
The survival rate and growth performance of M. rosenbergii fed diets supplemented with H. lacustris for 60 days are shown in Figs. 1 and 2. No significant differences in survival rate were observed among the groups (p>0.05) (Fig. 1). In contrast, growth performance, assessed by body weight and total length, showed significant differences between the H. lacustris-supplemented groups and the control (p<0.05). However, the magnitude of these growth differences was relatively small (Fig. 2).

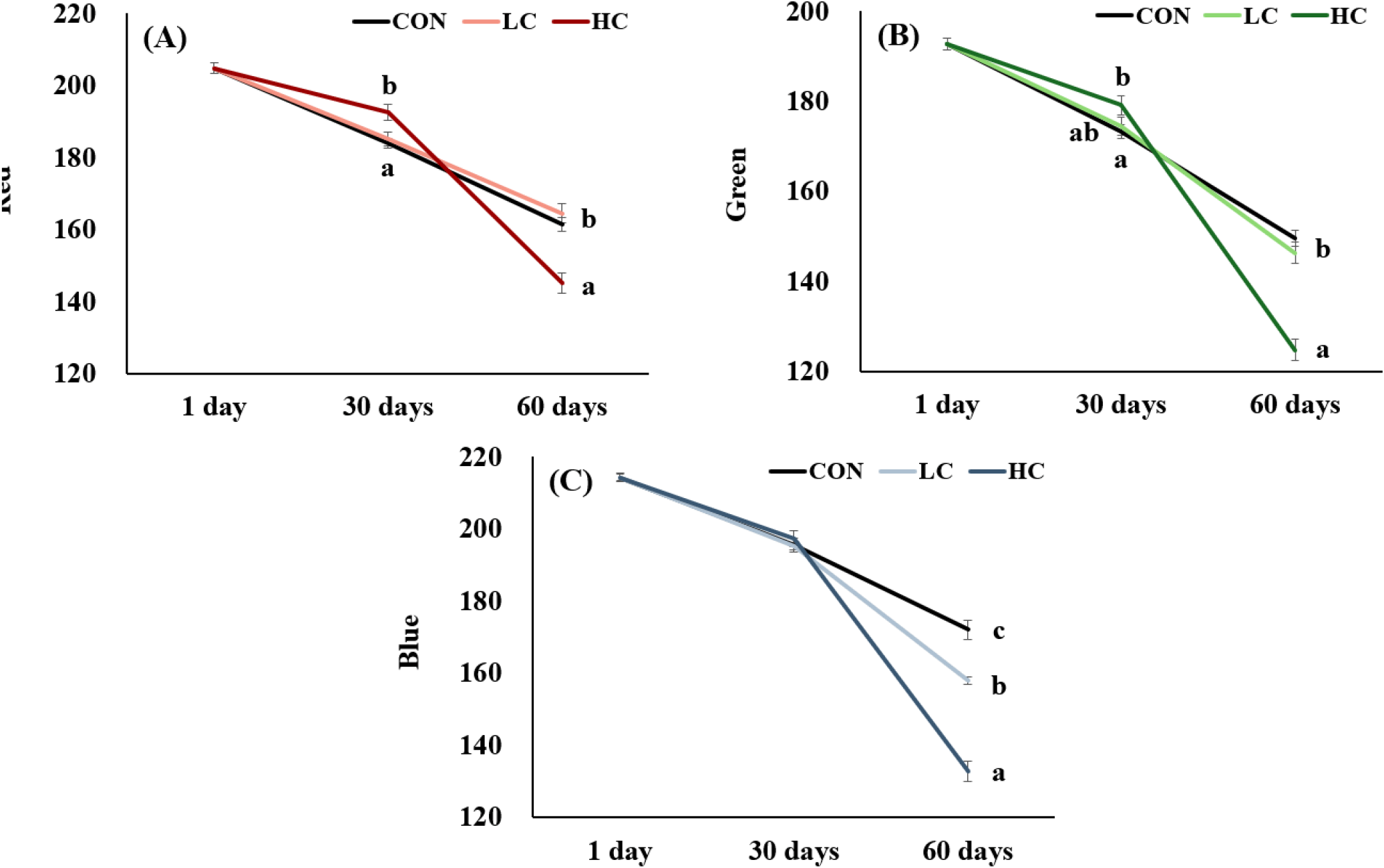
Analysis of body color using RGB values revealed that at 30 days, no significant differences were found between groups in the Green and Blue values, but HC showed significantly higher Red values compared to CON and LC groups. At 60 days, HC exhibited significantly lower Red and Green compared to CON and LC, while Blue values showed significant differences among all treatment groups (Fig. 3).
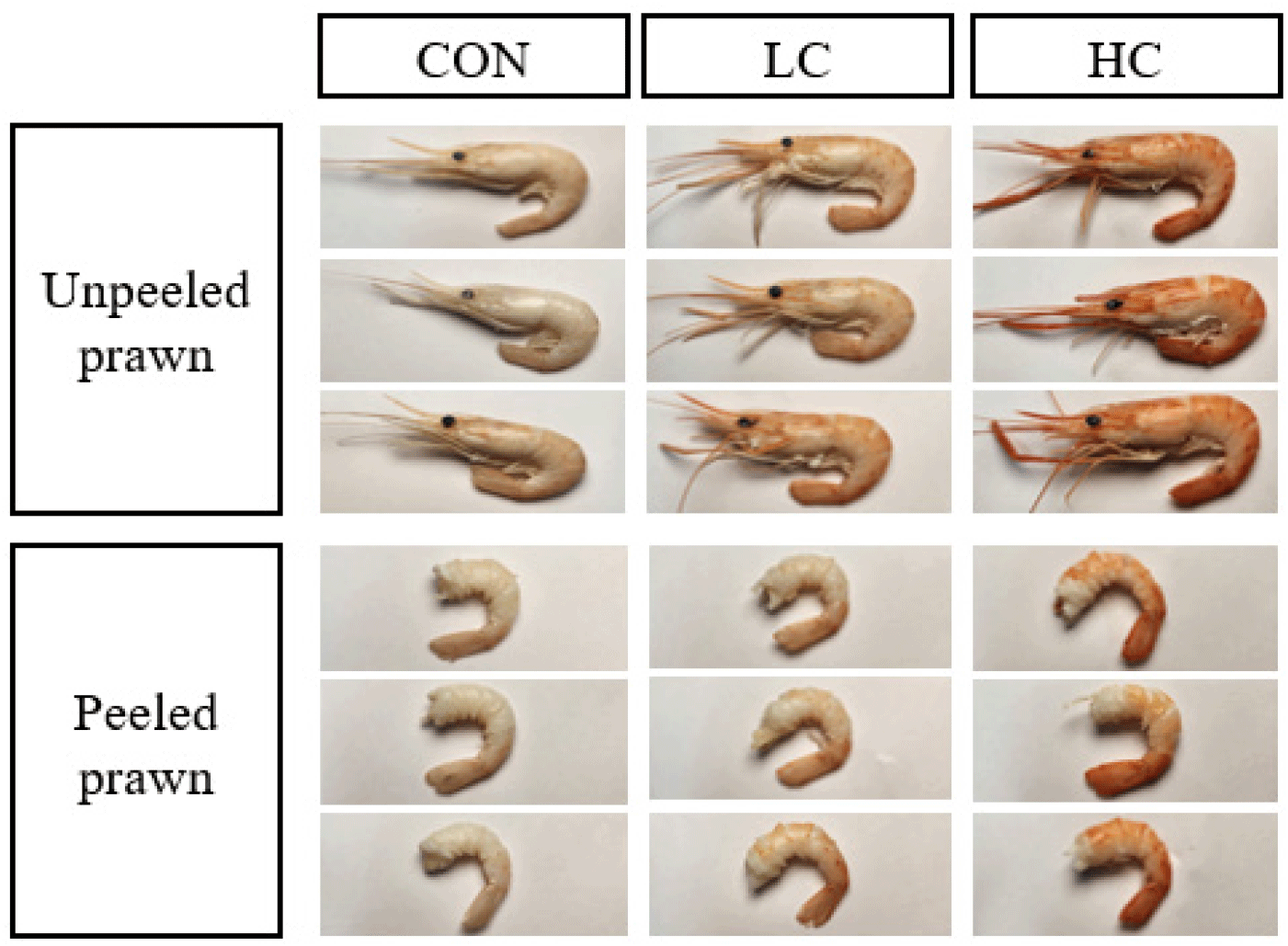
After heating in boiling water, the carapace and tail muscle of the CON turned a milky white, whereas prawns fed diets supplemented with H. lacustris developed a red coloration that increased in intensity with higher inclusion (Fig. 4).
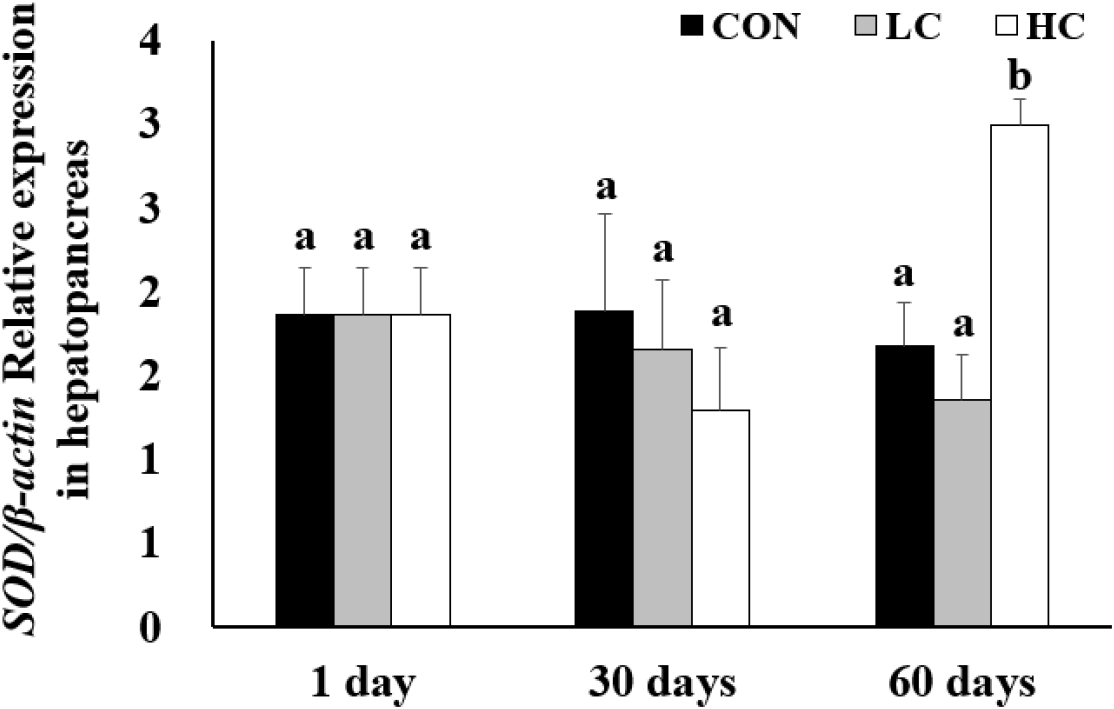
Analysis of SOD gene expression revealed no significant differences between groups on days 1 and 30. However, on day 60, expression levels in the HC group were significantly higher than those in the CON and LC groups (Fig. 5).
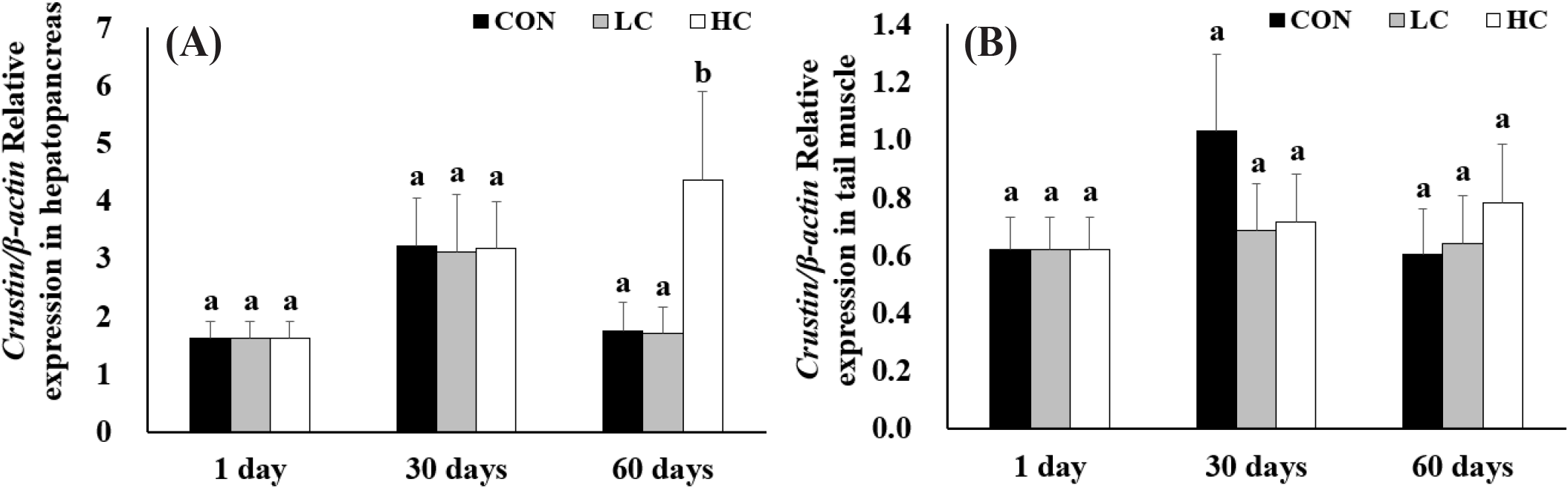
Crustin gene expression showed no significant differences among groups on days 1 and 30. However, by day 60, expression levels in HC group were significantly higher than those in the CON and LC groups. In the tail muscle, crustin expression showed no significant differences among all experimental groups throughout the entire period (Fig. 6).
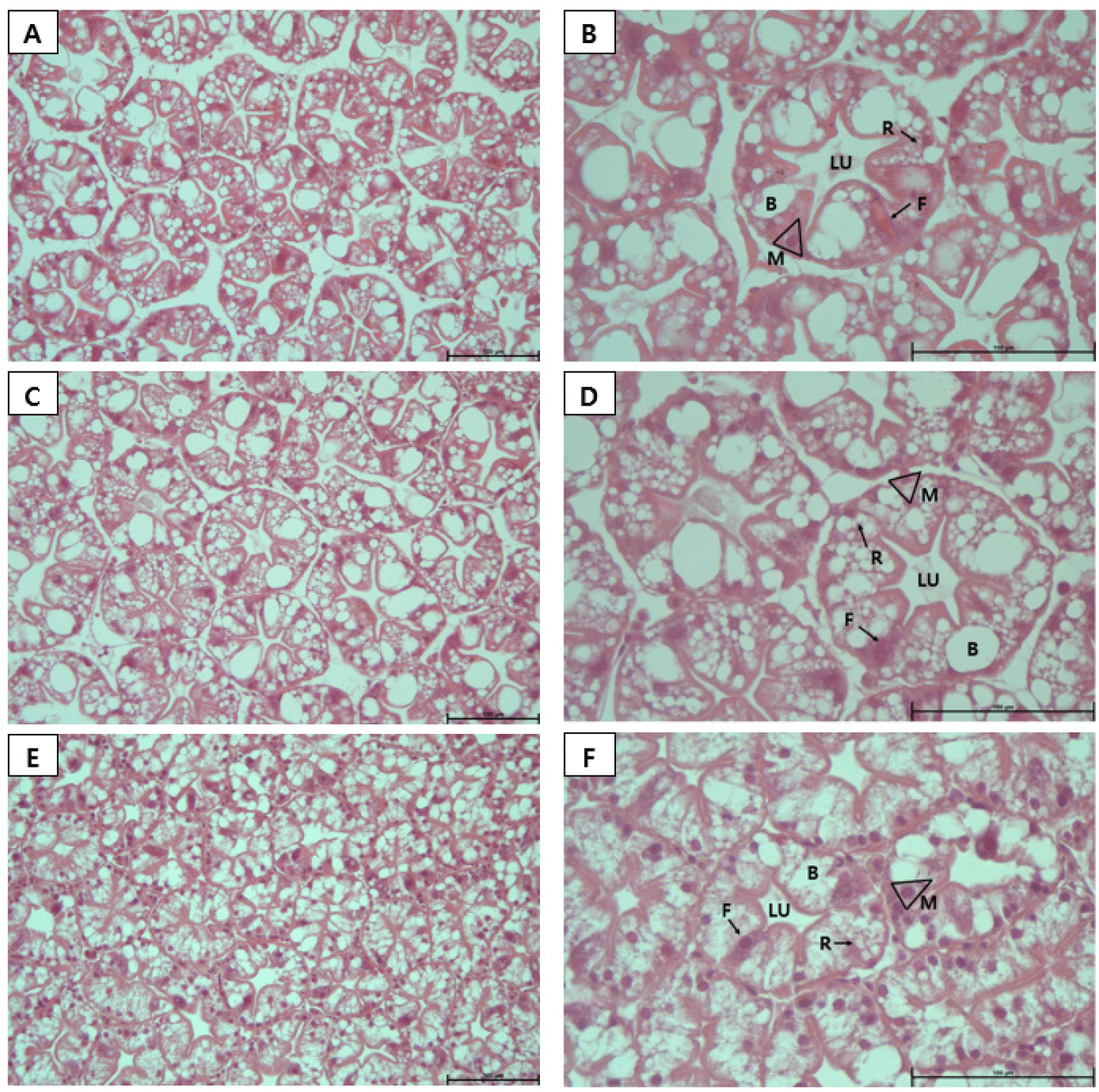
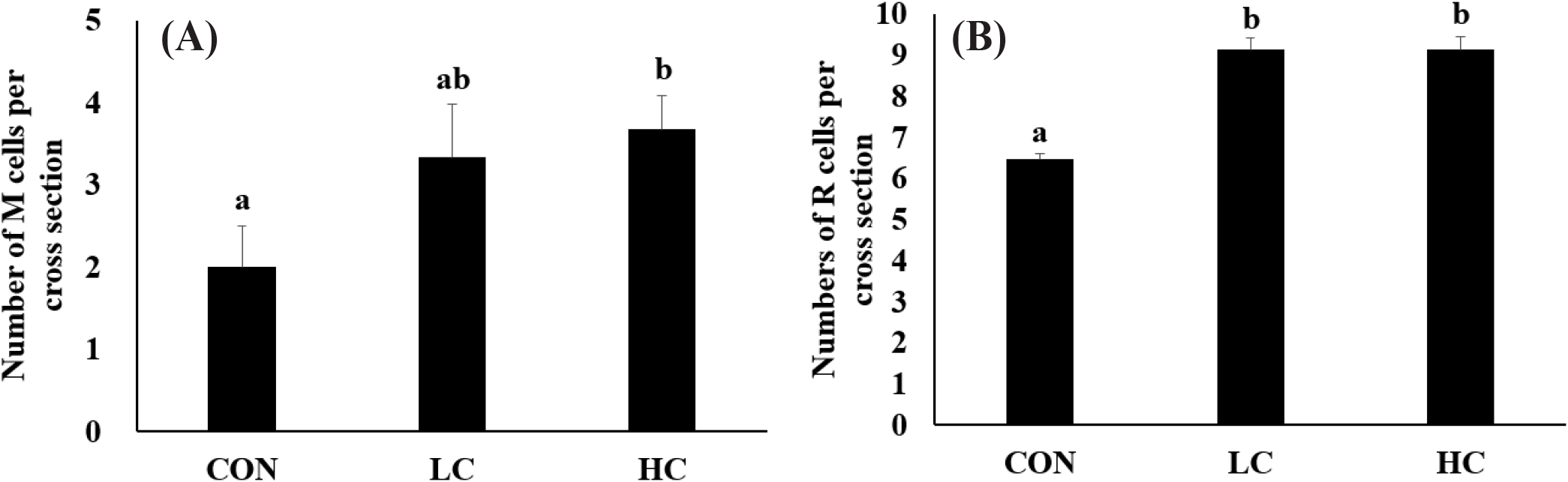
The hepatopancreas of all experimental groups maintained a nearly circular cross-sectional structure of the ducts. A marked increase in the number of R cells was observed in the H. lacustris treatment group, and in HC, granules within the R cells were notably more developed. In addition to the R cells, M cells also increased in number in proportion to the H. lacustris supplementation level (Figs. 7 and 8).
DISCUSSION
In this study, after feeding a diet supplemented with H. lacustris for 60 days, no significant differences were observed in the body weight to total length of M. rosenbergii. This finding is consistent with previous studies on L. vannamei fed H. lacustris (Xie et al., 2018) and on M. japonicus fed astaxanthin (Chien & Shiau, 2005), suggesting that supplementation with H. lacustris does not inhibit growth in M. rosenbergii. However, growth response may differ depending on the species, size, and developmental stage of the organisms, as well as the purification and processing methods used for H. lacustris (Wade et al., 2017; Xie et al., 2018).
The body color of crustaceans is a key quality indicator that strongly influences consumer purchasing decisions. In general, more vivid and intense coloration is associated with higher market value (Latscha, 1989). The low RGB values observed in the HC group in this study indicate deeper and more saturated coloration, demonstrating that the H. lacustris-supplemented diet effectively enhances the marketability of M. rosenbergii. This effect was particularly prominent at day 60, which is consistent with previous findings that astaxanthin accumulates gradually in the body over time and eventually influences pigmentation (Ju et al., 2011).
Crustaceans cannot synthesize carotenoids endogenously and must obtain them through their diet (Goodwin, 1952). Once ingested, carotenoids are metabolically transformed and used either for pigmentation or for various physiological functions. Because of these physiological characteristics, supplying H. lacustris as a carotenoid source is essential for pigment deposition and body color expression. Numerous studies have reported that astaxanthin has the strongest pigmentation effect among various carotenoids (Yamada et al., 1990; Chien & Jeng, 1992; Niu et al., 2012), which is consistent with the concentration-dependent enhancement observed in this study. In addition, the vivid red coloration that remained in the HC group even after heating indicates the thermal stability of astaxanthin (Parisenti et al., 2011). This suggests that the quality of pigmentation can be maintained during actual consumption and cooking processes.
SOD is a major antioxidant enzyme that eliminates ROS and plays an essential role in protecting tissues from cellular damage caused by oxidative stress and phagocytosis (Chien et al., 2003; Kim et al., 2019; Mansour et al., 2022). In this study, SOD gene expression in the hepatopancreas showed a decreasing trend on day 30, although the difference was not statistically significant. At day 60, expression levels were significantly higher in the HC group, suggesting that H. lacustris did not induce a positive antioxidant response. Similar results were observed in M. rosenbergii (Li et al., 2025) and Takifugu rubripes (Ou et al., 2019) when astaxanthin was supplied. when astaxanthin was provided. However, studies adding astaxanthin to L. vannamei (Zhang et al., 2013) and on H. lacustris supplementation (Fang et al., 2022) reported that astaxanthin-fed groups exhibited lower antioxidant gene expression than the controls, indicating that the optimal astaxanthin concentration may vary depending on species characteristics (Liao et al., 2018) also found that antioxidant enzyme activity in T. rubripes was significantly reduced at low astaxanthin concentrations, while no significant effect was observed at a high concentration of 500 mg/kg, suggesting that excessive intake may cause adverse effects.
Crustin is an immune-related peptide with antimicrobial activity in crustaceans (Smith et al., 2008), and its expression levels in the hepatopancreas also showed a significant increase. On day 60, HC showed significantly higher expression levels compared to CON and LC, suggesting that H. lacustris consumption induces immune gene expression. In contrast, no significant changes were observed in CON and LC, indicating that the effect occurred only at HC. No significant changes in crustin expression were detected in the tail muscle. This suggests that the tail muscle may be less sensitive to immune responses than the hepatopancreas, or that there may be tissue-specific differences in responsiveness to astaxanthin. Meanwhile, a study feeding Arthrospira platensis extract to L. vannamei (Mansour et al., 2022) reported a significant increase in crustin expression in the tail muscle as well, demonstrating that patterns of immune gene expression vary by tissue depending on experimental conditions.
The hepatopancreas integrates the functions of the pancreas, intestines, and liver to synthesize and secrete digestive enzymes, absorbing digested substances, and store nutrients (Caceci et al., 1988; NRC, 2011). It is composed of numerous hepatopancreatic tubules, each lined by a single layer of epithelial cells that include blister (B), fibrillary (F), reabsorption (R), embryonic (E), and midget (M) cell types (Franceschini-Vicentini et al., 2009). At the center of each villus lies the lumen, which serves as the passageway for the secretion of digestive enzymes and the transport of digested materials. Each cell type performs a distinct role, with E cells responsible for epithelial regeneration, F cells producing digestive enzymes and facilitating extracellular digestion, R cells storing lipids and glycogen, B cells carrying out intracellular digestion and nutrient absorption, and M cells involved in nutrient storage and endocrine regulation (Silva et al., 2018; Ruiz et al., 2020).
Histological analysis revealed that in all experimental groups, the hepatopancreas maintained structurally normal circular tubules and central lumens, with no evidence of tissue damage or pathological degeneration. However, clear differences were observed in cellular composition. While the distribution of B cells remained similar across all groups, HC showed significantly higher numbers of R cells compared to CON and LC, and these R cells contained numerous small vacuoles. R cells are known as the primary nutrient storage cells of the hepatopancreas (Vogt, 1994), and the findings of this study suggest that high-dose astaxanthin intake may have activated R cell function and increased their abundance (Wang et al., 2016). Lipid-soluble granules were particularly abundant in R cells in HC, suggesting that the high intake of astaxanthin, a lipid-soluble compound, may have increased both the number of R cells and intracellular lipid granule accumulation.
M cells also showed a similar increase in number. M cells are small cells located at the base of hepatopancreatic ducts (Ruiz et al., 2020). Positioned close to the duct’s basal region, they are small in size and do not extend into the duct lumen (Silva et al., 2018). In this study, the HC group exhibited an increase in the number of M cells. This is presumed to be due to the need to store large amounts of astaxanthin during digestion after ingesting high concentrations of H. lacustris, which may have increased the number of M cells responsible for nutrient storage.
CONCLUSION
This study investigated the effects of feed supplemented with the microalga H. lacustris on the growth performance, body coloration, and health status of M. rosenbergii. Over the 60 day rearing period, no significant difference in growth rate was detected among the treatments. A higher inclusion level of H. lacustris resulted in more pronounced body coloration, which is likely attributable to increased astaxanthin deposition in the tissues. The treatment also enhanced immune capacity by elevating crustin gene expression in the hepatopancreas. These findings altogether indicate that the addition of H. lacustris could help the body color development of M. rosenbergii post-larvae, suggesting that this approach may also be applicable to the body color development of various crustaceans.
