INTRODUCTION
Reproduction of fish is regulated by endocrine system through hypothalamus-pituitary-gonad axis and controlled by environmental factors such as water temperature and photoperiod (Modesto & Canario, 2003; Nagahama et al., 1994). Sex steroids from gonad play an important role during reproductive cycle and the relationship between reproductive cycle and plasma sex steroid hormones has been well documented in a number of teleosts including freshwater and marine species (Stequert et al., 2001; Larsson et al., 2002; Koya et al., 2003). Until now, estradiol-17β (E2), as a major sex steroid induces the vitellogenesis, vitellogenin uptake from liver to oocytes in females. After vitellogenesis, progestins such as 17α, 20β-dihydroxy-4-pregnen-3-one (17α20βP) and/or 17α, 20β,21-trihydroxy-4-pregnen-3-one (17α20β21P) induce final oocyte maturation including germinal vesicle breakdown (GVBD) and ovulation as the production of E2 decreases (Nagahama et al., 1994; Patiño & Sullivan, 2002). In males, testosterone (T) and 11-ketotestosterone (11KT) induce spermatogenesis and secondary sexual characteristics (Borg, 1994; Schulz et al., 2010).
Apart from sex steroid hormones, the representative corticosteroid, cortisol is produced from cholesterol by the adrenal cortex and increase in plasma cortisol levels is used as a major marker for stress responses (Bonga, 1997; Mommsen et al., 1999). Recently, Milla and colleagues reported that the levels of various corticosteroids including cortisol are higher during maturation stage than during immature stage in many teleost fish species; the authors suggested that corticosteroids may be actors of reproduction in several fish species (Milla et al., 2009). Moreover, there are some reports that increase in plasma cortisol levels during pre-spawning or spawning period although these results are controversial among teleost species (Kusakabe et al., 2003; Noaksson et al., 2005; Westring et al., 2008).
The ribbed gunnel, Dictyosoma burgeri is an intertidal teleost species distributed to coastal waters of Korea and Japan (Kim et al., 2001). After spawning, males guard spawned eggs attached to rocks (Shiogaki & Dotsu, 1972). Seasonal gonadal development and histological observation have been described in detail for the Jeju, Korea (Jin et al., 2007) however no information is available on seasonal changes of endocrine characteristics during annual reproductive cycle. The purpose of the present study was to investigate changes in plasma levels of T, E2, 17α20βP and cortisol from females and T, 17α20βP, 11KT and cortisol from males and obtain insights on their roles in annual reproductive cycle of ribbed gunnel.
MATERIALS AND METHODS
Adult male and female ribbed gunnels were collected by fishing trap in the coastal waters of Dadaepo, Busan, Korea from August 2002 to July 2003, except June 2003, due to problems of capture. And there were no males in March 2003. Fish were anesthetized in 0.1% 2-phenoxyethanol in seawater and blood was immediately collected from caudal artery with a heparinized 2 ml syringe. Blood samples were centrifuged at 4,000 g for 10 min, then plasma were collected and stored at –80°C until radioimmunoassay (RIA) was performed. Total length (cm) and standard length (SL, cm) was measured to nearest 0.01 cm. Body weight (BW, g) and gonad weight (GW, g) was measured to the nearest 0.01 g for calculation of gonadosomatic indices (GSI) according to GSI=100 ∙ GW ∙ BW–1.
Steroids from plasma were extracted twice in 2 ml diethyl ether, dried under nitrogen gas and resuspended in phosphate buffer (pH=7.5). Plasma E2, T, 17α20βP, 11KT and cortisol were measured by radioimmunoassay (RIA) according to the Kobayashi’s method (Kobayashi et al., 1987). Anti sera for E2, T, 17α20βP, 11KT and cortisol were purchased from Cosmo-Bio Co. Ltd. (Tokyo, Japan). Non-radioactive steroid standards were purchased from Steraloids Inc. (Wilton, NH, USA). Radiolabeled steroids ([3H]-T, [3H]-E2, [3H]-11KT and [3H]-cortisol) were purchased from Amersham Lifesciences (Piscataway, NJ, USA) and radio-labeled 17α20βP was prepared from [3H]-17α-hydroxyprogesterone (Amersham Lifesciences) by enzymatic conversion as described by Scott’s method (Scott et al., 1982) and separated from the parent compound by thin layer chromatography. The sensitivities of the assay were 12.5 pg/ml, 10 pg/ml, 16 pg/ml, 10 pg/ml and 22.5 pg/ml for E2, T, 11KT, 17α20βP and cortisol, respectively. The intra- and inter-assay coefficients of variations at 50% binding were 3.4% (n=3) and 11.5% (n=6) for E2, 2.3% (n=3) and 12.5% (n=6) for T, 3.9% (n=4) and 7.3% (n=8) for 11KT, 3.2% (n=4) and 9.5% (n=8) for 17α20βP and 2.8% (n=5) and 8.1% (n=6) for cortisol.
All data from steroid levels were expressed as means with the standard error of the means (SEM) and tested for normality using the Kolmogorov-Smirnov test using SPSS software (version 17.0) for Windows (SPSS, Chicago, IL, USA). However, the assumptions of normality and equal variance were failed, and then non-parametric Kruskal-Wallis test was performed and, when significant, followed by Bonferroni’s adjustment for comparison of monthly mean values of plasma steroid levels. A value of P<0.05 was considered statistically significant. In the correlation analysis among plasma steroid levels, we examined Spearman’s rank correlation test followed by multiple comparisons with each steroid levels. Statistical significance was set at α=0.05.
RESULTS
During the sampling period, 66 females and 67 males were captured for this study and monthly distribution of their SL and BW are listed in Table 1. GSI of females increased since November, peaked in February (11.10±2.74) and then decreased rapidly in March (1.05±0.23, Fig. 1). In males, GSI increased since November, peaked in January (0.46±0.25), then decreased gradually until April (0.15±0.05) and remained at a low level until July (0.05±0.01~0.07±0.01).
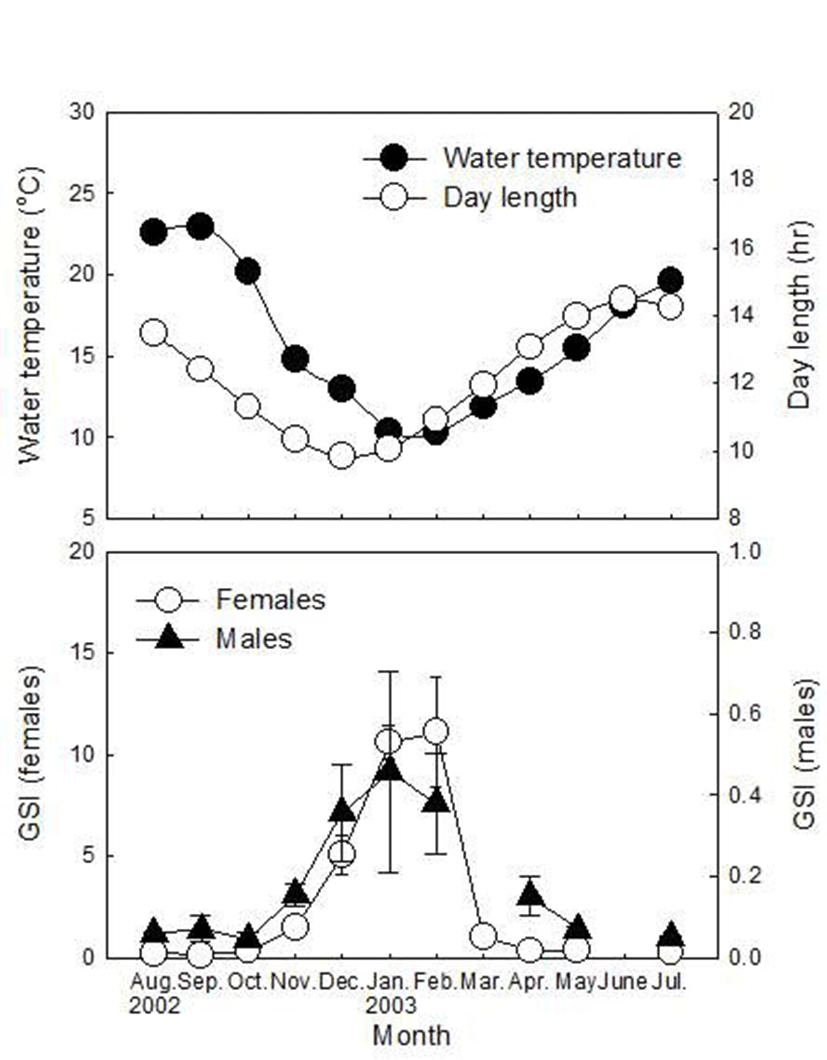
Plasma T levels of females were low in August and September (0.95±0.06 and 0.87±0.07 ng/ml, respectively), increased from November (2.74±0.58 ng/ml) and peaked in January (13.12±6.46 ng/ml, Fig. 2). T levels then decreased rapidly in March (0.64±0.11 ng/ml) and remained at a low level until July (0.64±0.08~1.41±0.21 ng/ml). Plasma E2 levels of females were low from August to November (1.28±0.25~3.03±0.68 ng/ml) and increased rapidly and remained at a high level from December to February (19.81±7.46~22.85±6.18 ng/ml). E2 levels then decreased rapidly in March (0.53±0.09 ng/ml) and remained at a low level until July (0.78±0.06~2.06±0.80 ng/ml). Plasma 17α20βP levels of females were low from August to January (1.23±0.22~2.93±0.35 ng/ml), increased rapidly from February (4.78±1.01 ng/ml) and remained at a high level in March (4.15±2.02 ng/ml). Then, 17α20βP levels decreased until April (2.01±0.12 ng/ml) but increased in July (5.08±0.65 ng/ml) as the highest level. Plasma cortisol levels of females fluctuated from August to December (221.33±65.90~1,017.87±351.52 ng/ml), began to increase in January and peaked in March (2,081.07±1,140.02 ng/ml). Cortisol level decreased rapidly in April (386.56±70.50 ng/ml) and increased again in May (666.93±150.44 ng/ml) and July (1,525.76± 446.93 ng/ml).
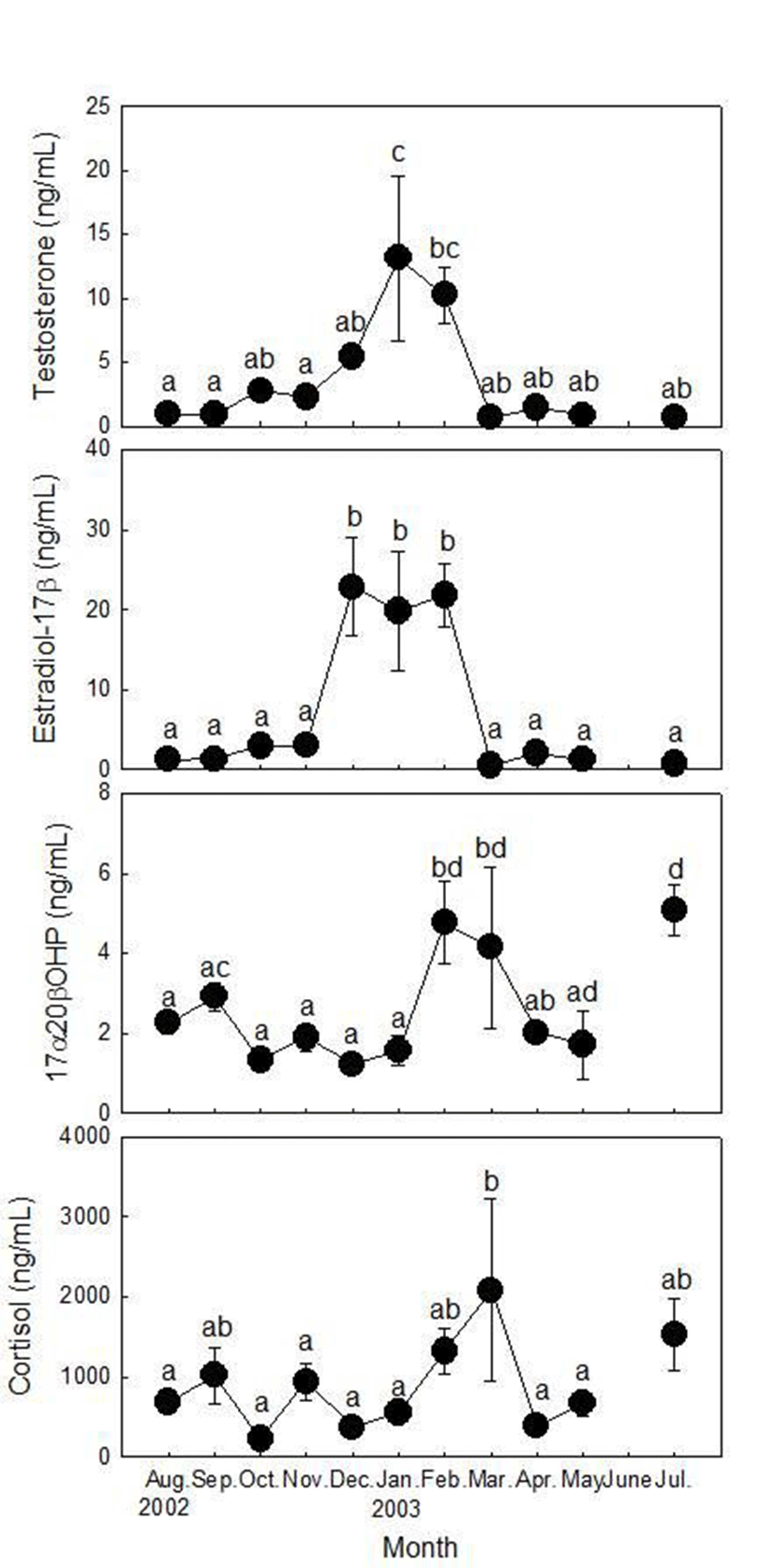
There were highly significant correlations between plasma T and E2 levels (rs=0.898, p<0.01, Spearman rank correlation), between plasma 17α20βP and cortisol levels (rs=0.696, p<0.01, Spearman rank correlation) in female ribbed gunnel (Fig. 4).
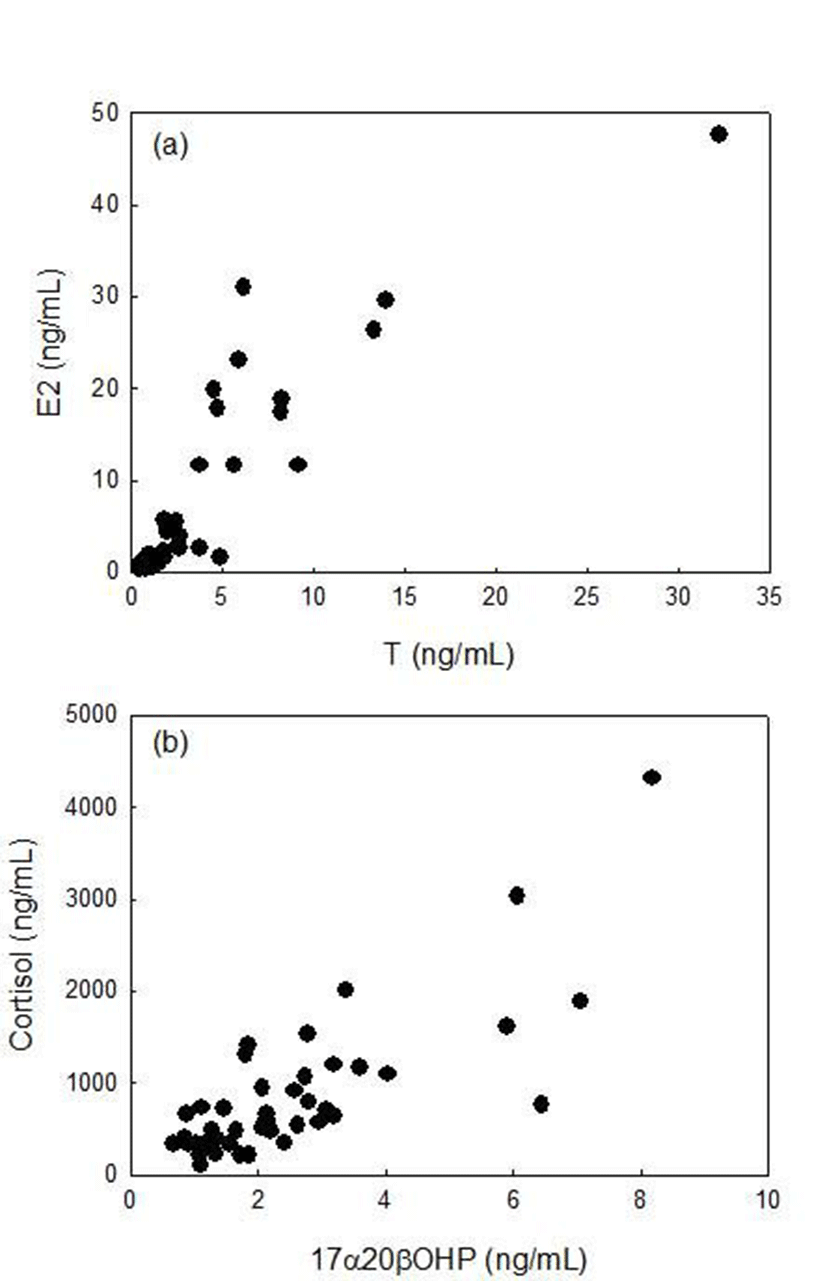
Plasma T levels of males increased from September to November, decreased in December and peaked in January (2.43±0.17 ng/ml, Fig. 3). T levels decreased rapidly in February (1.25±0.48 ng/ml) and remained low levels in May and July (0.85±0.12 and 0.91±0.05 ng/ml, respectively). Plasma 17α20βP levels were low in August (1.95±0.53 ng/ml), increased rapidly in September (4.35± 0.31 ng/ml) and decreased gradually until December (1.72±0.12 3.38±~ 1.00 ng/ml). Then 17α20βP levels increased again in January (2.76±1.42 ng/ml) and February (4.11±0.78 ng/ml). 17α20βP levels were low in April (1.70±0.23 ng/ml) and peaked in May (4.67±0.66 ng/ml). In July, 17α20βP levels decreased again (3.84±0.72 ng/ml). Plasma 11KT levels were low in August and September (1.12±0.31~1.45±0.22 ng/ml) and maintained high levels from October to January (3.27±1.55~4.28±1.53 ng/ml). Then, 11KT levels decreased and maintained low levels from February to July (0.56±0.08~1.29±0.27 ng/ml). Plasma cortisol levels were high in August and September, decreased in October as the lowest level (323.20±18.40 ng/ml). Cortisol levels remained at high levels from November to February (647.60±211.92~ 1,102.67±339.03 ng/ml) and decreased in April (479.80± 238.28 ng/ml). Then, cortisol levels increased again in May and peaked in July (1,794.40±509.09 ng/ml).
DISCUSSION
The spawning periods of ribbed gunnel were considered from January to February; 17α20βP levels of females and GSI of both females and males were high during this period. E2 levels of female ribbed gunnel were high from December to February during spawning period and were very low throughout most of the year. These were coincident with T levels and these two steroids were significantly positively correlated. Similar results have been reported in other fish species (Mori et al., 2003; García-López et al., 2006). In general, plasma E2 levels increase during vitellogenesis and decrease during final oocyte maturation stage by production of progestins such as 17α20βP or 17α20β21P in many teleost species (Nagahama et al., 1994; Patiño & Sullivan, 2002). Although the role of T on reproduction of female fish was not identified clearly, the correlation between T and E2 has been attributed to the role of T as a precursor to E2 (Shiogaki & Dotsu, 1972; Matsuyama et al., 1988). However, plasma T and E2 levels from female ribbed gunnel were maintained highly in February when GSI was the highest value and 17α20βP began to increase. In the view of sexual behavior, ribbed gunnel is nestbuilding species (Shiogaki & Dotsu, 1972). Desjardins and colleagues also reported that females are more aggressive than males and have higher plasma T levels than their mating males, suggesting a role for T in female aggression (Desjardins et al., 2008). The results for high levels of T during spawning period suggest that T may promote aggressive and nest-building behavior in female ribbed gunnel although the role of T in this aspect of female has not been investigated. In Eurasia perch, Perca fluviatilis, plasma E2 levels also increased continuously prior to GVBD and decreased sharply after GVBD (Fontain et al., 2003; Migaud et al., 2003). In addition, our previous study reported that synthesis of E2 metabolites increased as oocyte diameter increased in mature oocytes (germinal vesicle migratory stage) from ribbed gunnel (Hwang & Baek, 2010). Therefore, we suspect E2 may regulate not only vitellogenesis but final maturation process in ribbed gunnel although further studies should be conducted.
Plasma levels of 17α20βP from females began to increase in February and then decreased until May. We consider 17α20βP may be a maturation inducing steroid in this species with comparison of changes in GSI. Interestingly, the highest level of 17α20βP was detected in July although there was a high value in spawning period. In July, GSI and both of T and E2 levels were low. The reason is not clear yet. The action of 17α20βP may be involved in other physiological role rather than reproduction in July. According to Antonopoulou and colleagues, 17α20βP may have a novel hormonal activity such as stimulating meiotic division of the next year’s gametes (Antonopoulou et al., 2011). In a recent study, Jin and colleagues reported that the ovarian developmental stage of ribbed gunnel in July was recovery stage; most ovarian follicles consist of oocytes at the perinucleolous stage (Jin et al., 2007). Therefore, the peak of 17α20βP level in July would be explained as possible regulation of 17α20βP on the meiotic division of oocyets. However, we should conduct histological observation of ovary and other markers for meiotic division as suggested by Miura and colleagues (Miura et al., 2007). Meanwhile, it is also possible to consider that 17α20βP is produced at other tissue besides ovary. Interrenal and gills are capable of producing 17α20βP in vitro and the presence of 20β-hydroxysteroid dehydrogenase (HSD), a crucial enzyme for conversion to 17α20βP were identified (Sangalang & Freeman, 1988; Ebrahimi et al., 1996; Hoffmann & Oris, 2006). However, we have insufficient evidence at this point since we did not confirm the production of 17α20βP from gills or interrenal tissues. Conclusively, we suspect that the peak of 17α20βP level in July which four months after spawning season will be related to meiotic division of ovarian development.
In male ribbed gunnel, GSI increased since October and was the highest value in January and plasma T levels were also the highest value in January. 11KT levels were high from October to January with fluctuations and then decreased until July. However, 17α20βP levels decreased from September until December and then increased until February although there were high fluctuations during annual period. In general, plasma levels of T and 11KT are the highest during spermatogenesis in most teleost species and 17α20βP is the highest at during spermiation (Jensen et al., 2001; Scott et al., 2010). In the present study, however, there was no significant correlation between T and 11KT as reported by other fish species (Jensen et al., 2001, Mori et al., 2003). Nevertheless, levels of T and 11KT were maintained high levels during period when GSI was high, and these results suggested that both of T and 11KT may regulate spermatogenesis in ribbed gunnel. On the other hand, changes in plasma levels of 17α20βP were quite different with changes of T or 11KT. Although 17α20βP levels increased from December to February, higher levels than this period were observed in September and May. The reason for high levels of 17α20βP in September, May and July is remained unclear. Future studies should be conducted with any other physiological role of 17α20βP on males in this period.
In the present study, there was a significantly positive correlation between 17α20βP and cortisol levels from female ribbed gunnel although there was no periodical change in cortisol levels from males. Moreover, cortisol levels from both females and males were also high in July when 17α20βP levels were also high. These results suggested that cortisol may be involved in reproduction of ribbed gunnel. Classically, cortisol is mainly produced from interrenal tissue. Other studies reported that cortisol is also produced from ovary (Kusakabe et al., 2002; Kazeto et al., 2003; Zhou et al., 2007). These studies support the presence of corticosteroidogenesis in ovary. In earlier previous study, Mommsen and colleagues demonstrated that cortisol may suppress reproductive function; cortisol suppressed vitellogenin levels in plasma and vitellogenin synthesis from liver (Mommsen et al., 1999). However, Milla and colleagues reported that cortisol may involve in fish reproduction positively; the authors indicated that higher cortisol levels in mature stage than immature stage were reported with several fish species (Milla et al., 2009). In addition, cortisol could induce in vitro oocyte maturation followed volume increase by hydration and increase the expression of glucocorticoid receoptor (Milla et al., 2006). There also has been controversy in the function of cortisol in reproduction in other vertebrates. For examples, cortisol exhibited a positive action during ovulation in human (Jimena et al., 1992; Andersen, 2002) although it had no significant effect on oocyte maturation of mouse or it inhibited oocyte maturation of pig (Yang et al., 1999; Andersen, 2003). However, the present data are sufficient to evoke the functional action of cortisol on reproduction even though there are still controversies and we do have insufficient data about it.
In conclusion, changes in sex steroid levels in ribbed gunnels have annual periodicity and we suspect that 17α20βP may regulate early oocyte development. Moreover, cortisol may involve in maturation process and act as an index for not only in stress response but in reproduction of ribbed gunnel.