ARTICLE
Intersexuality of Scomberomorus niphonius from the Coastal Area around Jeju Island, Korea (Teleostei: Scombridae)
Jong Bin Kim1, Dae Soo Chang1, Hyejin Kim2, Mi Ae Jeon2, Kayeon Ku2, Jung Sick Lee2,†
†Corresponding author: Jung Sick Lee, Department of Aqualife Medicine, Chonnam National University, Yeosu 550-749, Republic of Korea. Tel. :+82-61-659-7172, Fax : +82-61-659-7170, E-mail:
ljs@chonnam.ac.kr
Copyright © 2013 © Copyright A Official Journal of the Korean Society of Developmental Biology. All Rights Reserved.. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: Mar 26, 2013 ; Revised: Apr 17, 2013 ; Accepted: Apr 24, 2013
ABSTRACT
This study histologically describes the intersexuality of Scomberomorus niphonius collected from the coastal area near Jeju Island. A total of 126 S. niphonius, collected from March to July 2012 with a total length of 62.4 cm (±17.5) and a total weight of 1,701.9 g (±1,528.9) were used for analyses. From a histological perspective, two types of intersex were confirmed. One type had scattered germ cells from the opposite sex within the gonad. The second type developed germ cells from the opposite sex in the connective tissue of the outer gonadal membrane. The intersexuality was 14.3% (n=18/126), with females (21.3%; n=16/75) exhibiting a higher rate than males (3.9%; n=2/51). There was no displayed correlation between intersexuality and the total length and weight.
Keywords: Intersexuality; Scomberomorus niphonius.
INTRODUCTION
In an aquatic ecosystem, the environmental risk assessment of living things needs to be researched to keep the ecosystem healthy and to aid in its recovery (Tayler et al., 1998; Lee et al., 2010).
There are four main steps in the risk assessment for environmental factors: 1) hazard identification, 2) dose-response assessment, 3) exposure assessment, and 4) risk characterization. The first method is for monitoring general changes in an ecosystem (NRC, 1983).
A biomarker refers to a cell indicator or an individual physiology that detects exogenous factors affecting a living organism, biochemistry, and structure. Among the physiological biomarkers, reproductive indexes are an important point to detect long-term and continuous effects of pathogenic processes (Huggett et al., 1992).
Chemical water pollutants are generally divided into three categories; heavy metals, persistent organic pollutants (POPs), and endocrine disrupting chemicals (EDCs). EDCs disturb the reproductive endocrine system and change the manifestation or function of sex in aquatic animals, as either androgenic or estrogenic effectors. EDCs mimic sex hormones and may cause aberrant outcomes including reproductive, infertility problems, and intersex in wildlife (Tyler & Routledge, 1998; Kwon et al., 2006; Ju et al., 2009; Lee et al., 2009).
This study is to analyze the intersex characteristics depending on sex and size of Scomberomorus niphonius collected from the coastal area near Jeju Island.
MATERIALS AND METHODS
1. Materials
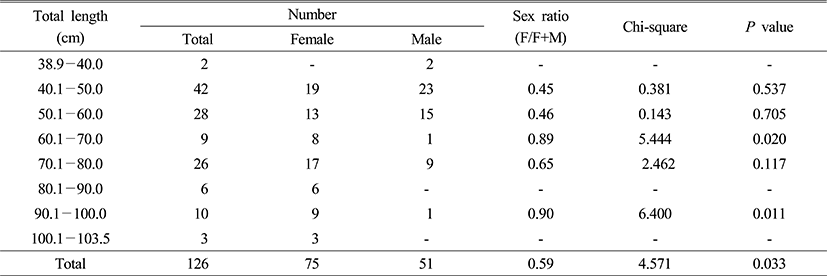
The 126 specimens of S. niphonius, with a total length of 62.4 cm (±17.5) and a total weight of 1,701.9 g (±1,528.9), were collected from the coastal area near Jeju Island in Korea by mid water trawling from March to July 2012 (Table 1).
2. Methods
1) The sex ratio and intersex
The sex ratio and intersex ratio were categorized by gonadal specimen. Intersex includes only when the opposite sex germ cell is observed but no other sex characteristic.
2) The histological analysis
The experimental fish were measured with their total length and weight after being checked for any defects. Gonads were removed and weighed to the nearest 0.1 g using digital calipers. The tissue samples were fixed in Bouin's solution for 24 hours and washed in running water for 36 to 48 hours. The samples were then prepared in paraffin sections after being dehydrated with alcohol. The sections were cut at 4 to 6 μm with a microtome. The resulting sections were placed on slides, stained with Mayer's hematoxylin-eosin (H-E), and observed under a microscope.
RESULTS
1. The histological characteristics of intersex
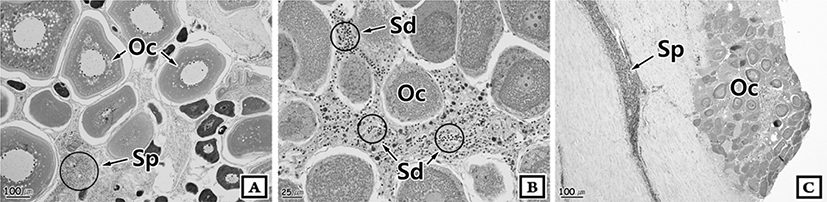
From the histological perspectives, two types of intersex were observed. The first type shows the opposite germ cells scattered randomly throughout the gonad, which were found in all females (Fig. 1A, B). The second type shows mature germ cells from the opposite sex in the connective tissue of the outer gonadal membrane, which were found in all males (Fig. 1C). Intersex fish are seen to possess degenerated oocyte in their ovary (Fig. 1B) and previtellogenic oocyte in the intersex testis (Fig. 1C).
Fig. 1.
Photomicrograph of intersex gonad of Scomberomorus niphonius. A and B, Female; C, Male. Oc: Oocytes, Sd: Spermatids, Sp: sperm.
Download Original Figure
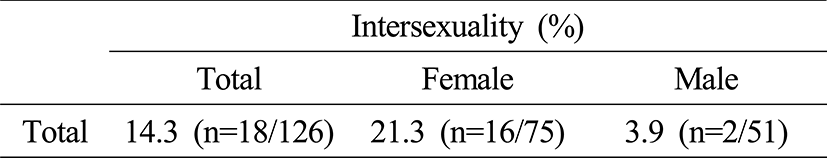
2. Sex and intersexuality
14.3% (n=18/126) of intersex fish were found in total. Intersexuality of the female and male was 21.3% (n=16/75) and 3.9% (n=2/51) respectively; it was indicated female higher than males (Table 2).
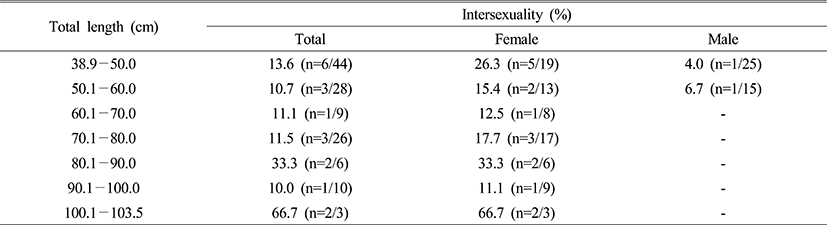
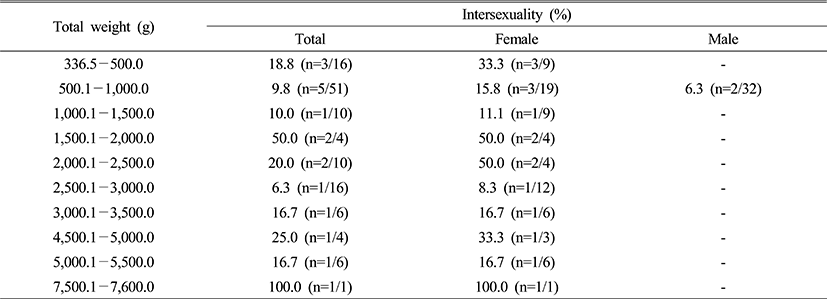
3. Size and intersexuality
Collected specimens were measured and classified every 10.0 cm for the total length and 500.0 g for the total weight. Intersexuality in accordance with the total length and weight did not display apparent correlation (Table 3 and 4).
DISCUSSION
Morphological sex character in teleost is fundamentally genetic like in other vertebrate, but environmental factors at the early life stages can influence sex differentiation of male and female (Devlin & Nagahama, 2002).
Reproductive biological characteristics are mainly used to understand how the marine environment affects fish on the population level. Among various reproductive biological characteristics, being intersex is a stable and long-term indicator. Thus, the intersexuality analysis provides useful monitoring on sensitive aquatic ecosystem indicating chemical stressors of chemical pollutants including endocrine disrupting chemicals (EDCs) (Drysdale & Bortone, 1989; Borton et al., 1991; Huggett et al., 1992; Bortone & Davis, 1994; Jobling et al., 1998, 2002; Vigano et al., 2001; Lee et al., 2010).
Hermaphroditism is found in about 400 fish species, which are mostly tropical or subtropical. Generally hermaphroditism in fish occurs if male gonadal tissues is increased, and the opposite sex gonadal tissues degenerate. The gonad structure of hermaphrodite fish was divided into delimited and undelimited type. In the delimited type the testicular tissue and ovarian tissue are divided by a membrane of connective tissue. In the undelimited type the testicular tissue and ovarian tissue are divided, but 1) the connective membrane does not exist and 2) the testicular tissue and ovarian tissue are mixed (Sadovy & Shapiro, 1987). The two types of histological intersex are identified in the present study. These kinds of histological intersex are similar to Acanthogobius flavimanus, Chelon haematocheilus, Hemibarbus labeo, Leiognathus nuchalis, Mugil cephalus, and Synechogobius hasta (Lee et al., 2010).
The exposure of fish to EDCs can cause sexual development disorders such as feminizing of males or masculine effects on females in an endocrine system of aquatic animals (Gimeno et al., 1998a, b; Iguchi, 1998; Ackermann et al., 2002; Metrio et al., 2003; Quinn et al., 2004). The masculine effects of EDCs are reported in tributyltin (TBT), polychlorinated biphenyls (PCBs), and zinc (Holm et al., 1991; Matta et al., 1998; Ju et al., 2009; Lee et al., 2009). Phenols are known for one of the EDCs that feminize males (Gimeno et al., 1997; Gray & Metcalfe, 1997; Gray et al., 1999).
Alkylphenols, a type of EDCs, induce developing an oviduct to Cyprinus carpio in genetically male fish (Gimeno et al., 1997). Estrogens and alkylphenols do the same to Rutilus rutilus males (Trevor et al., 2001). PCBs are inhibited to ovarian development in the sex differentiation of Oncorhynchus mykiss (Matta et al., 1998). They also induce intersexuality to Scaphirhynchus albus males (Harshbarger et al., 2000). Bis-tributyltin oxide (TBTO) causes Gasterosteus aculeatus degeneration of the oocytes (Holm et al., 1991). Nonylphenols and octylphenols causes intersexuality to male medaka (Oryzias latipes) (Gray & Metcalfe, 1997; Gray et al., 1999), and the degeneration of the oocytes or sperm maturation to zebra fish (Danio rerio) (Weber et al., 2003).
Intersexuality, caused by EDCs, in South Korea was noticed in teleost including Acanthogobius flavimanus, Chelon haematocheilus, Hemibarbus labeo, Leiognathus nuchalis, Mugil cephalus, and Synechogobius hasta collected near costal area near industrial cities in Ulsan, Onsan, Shihwa, Anshan, Yeosu, and Gwangyang (Lee et al., 2010).
This study found 14.3% of intersexuality in Scomberomorus niphonius, whereas females were 21.3% and males were 3.9% showing females have a higher intersex rate than males. The exposure of fish to EDCs that masculinize female fish is known to be the cause, but further studies in detail are necessary.
The effect that EDCs have on ecology varies according to the species, age, and life stage of fish (Niimi, 1983). These chemicals are mostly lipid-soluble, which allows great persistence within the ecosystem, and thus medium levels of transportation and biological accumulation through the food chain (Longnecker et al., 1997; Nilsson, 2000; Safe et al., 2000). Even when the EDCs are at low concentrations, they can have a harmful influence on humans or other animals that are at higher levels of the food web (Tyler et al., 1998; Trevor et al., 2001).
However, this analysis showed no correlation between intersexuality to the total length and weight of Scomberomorus niphonius. These results could be due to a temporary exposure to EDCs than EDCs accumulation throughout the food web or continuous exposure. This study was limited to the hazard identification stage, which is an environmental risk assessment stage suggested by the NRC (1983). In order to find out more about the pollutants and the causal relationship identified in this study, further research is needed.
ACKNOWLEDGMENTS
This work was funded by a grant from the National Fisheries Research & Development Institute (NFRDI, RP-2012-FR-049), Korea.
REFERENCES
Ackermann GE, Schwaiger J, Negele RD, Fent K. Effects of long-term nonylphenol exposure on gonadal development and biomarkers of estrogenicity in juvenile rainbow trout
Oncorhynchus mykiss. Aquat Toxicol. 2002; 6:203-221.

Borton D, Carroll S, Goldberg S, Harrington K, Seltzer B, Dikon A. Relationship between severity of illness and nosocomial infection: A trending model. Am J Infect Control. 1991; 1:123.

Bortone SA, Davis WP. Fish intersexuality as indicator of environmental stress: monitoring fish reproductive systems can serve to alert humans to potential harm. Bio Science. 1994; 4:165-172.

De Metrio G, Corriero A, Desantis S, Zubani D, Cirillo F, Deflorio M, Bridges CR, Eicker J, de la Serna JM, Megalofonou P, Kime DE. Evidence of a high percentage of intersex in the Mediterranean swordfish
Xiphias gladius L. Mar Pollut Bull. 2003; 46:358-361.

Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002; 208:191-364.

Drysdale DT, Bortone SA. Laboratory induction of intersexuality in the mosquitofish
Gambusia affinis, using paper mill effluent. Bull Environ Contam Toxicol. 1989; 43:611-617.

Gimeno S, Komen H, Gerritsen AGM, Bowmer T. Feminisation of young males of the common carp
Cyprinus carpio, exposed to 4-
tert-pentylphenol during sexual differentiation. Aquat Toxicol. 1998; 43:77-92.

Gimeno S, Komen H, Jobling S, Sumpter J, Bowmer T. Demasculinisation of sexually mature male common carp
Cyprinus carpio, exposed to 4-
tert-pentylphenol during spermatogenesis. Aquat Toxicol. 1998; 43:93-109.

Gimeno S, Komen H, Venderbosch PWM, Bowmer T. Disruption of sexual differentiation in genetic male common carp (
Cyprinus carpio) exposed to an alkylphenol during different life stages. Environ Sci Technol. 1997; 31:2884-2890.

Gray MA, Metcalfe CD. Induction of testis-ova in Japanese medaka
Oryzias latipes exposed to
p-nonylphenol. Environ Toxicol Chem. 1997; 16:1082-1086.

Gray MA, Niimi AJ, Metcalfe CD. Factors affecting the development of testis-ova in medaka
Oryzias latipes, exposed to octylphenol. Environ Toxicol Chem. 1999; 18:1835-1842.

Harshbarger JC, Coffey MJ, Young MY. Intersexes in Mississippi River shovelnose sturgeon sampled below Saint Louis, Missouri, USA. Mar Environ Res. 2000; 50:247-250.

Holm G, Norrgren L, Linden O. Reproductive and histopathological effects of long-term experimental exposure to bis(tributyltin)oxide (TBTO) on the three-spined stickleback
Gasterosteus aculeatus Linnaeus. J Fish Biol. 1991; 38:373-386.

Huggett RJ, Kimerle RA, Mehrle PM, Bergman HL. Biomarkers - Biochemical, Physiological, and Histological Markers of Anthropogenic Stress. 1992; London: Lewis Publishers. p. 1-347.

Iguchi T. Environmental endocrine disruptors. Jap J Clin Med. 1998; 56:2953-2962.

Jobling S, Coey S, Whitmore JG, Kime DE, Van Look KJW, McAllister BG, Beresford N, Henshaw AC, Brisghty G, Tyler CR, Sumpter JP. Wild intersex roach
Rutilus rutilus have reduced fertility. Biol Reprod. 2002; 67:515-524.

Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. Widespread sexual disruption in wild fish. Environ Sci Technol. 1998; 32:2498-2506.

Ju SM, Park JJ, Lee JS. Induction of intersex and masculinization of the equilateral venus
Gomphina veneriformis (Bivalvia: Veneridae) by zinc. Ani Cell Sys. 2009; 13:339-344.

Kwon JY, Lee CH, Kim JY, Kim SH, Kin DJ, Han HK, Lim HK, Byun SG. Disruption of sex differentiation by exogenous sex steroid hormones in Korean rockfish,
Sebastes schlegeli. Dev Reprod. 2006; 10:247-254.

Lee JS, Kim JW, Park JJ, Ju SM, Park JS, Lee DG, Yun TW, Choi K, Yoon J, Eom I. Sex ratio and intersexuality in coastal fishes near industrial complex of Korea. J Fish Pathol. 2010; 23:211-219.

Lee JS, Cho HS, Jin YG, Park JJ, Shin YK. Reproductive disrupting effect of organotin compound in the ark shell
Scapharca broughtonii (Bivalvia: Arcidae). Ani Cell Sys. 2009; 13:223-227.

Longnecker MP, Rogan WJ, Lucier G. The human health effects of DDT (dichlorodiphenyl-trichloroethane) and PCBs (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu Rev Public Health. 1997; 18:211-244.

Matta MB, Cairncross C, Kocan RM. Possible effects of polychlorinated biphenyls on sex determination in rainbow trout. Environ Toxicol Chem. 1998; 17:26-29.

Nilsson R. Endocrine modulators in the food chain and environment. Toxicol Pathol. 2000; 28:420-431.

NRC (National Research Council) Risk Assessment in the Federal Government: Managing the Process. 1983; Washington, D.C: National Academy Press. p. 1-192.

Quinn B, Gagne F, Costello M, McKenzie C, Wilson J, Mothersill C. The endocrine disrupting effect of municipal effluent on the zebra mussel (
Dreissena polymorpha). Aquat Toxicol. 2004; 66:279-292.

Sadovy Y, Shapiro DY. Criteria for the diagnosis of hermaphroditism in fishes. Copeia. 1987; 1987:136-156.

Safe SH, Welsch F, Janszen DB. Endocrine disruptors and human health: Is there a problem? An update. Environ Health Perspect. 2000; 108:487-493.

Trevor PRG, Susan J, Carole K, Steven M, Geoff B, Michael JW, John PS, Charles RT. Exposure of juvenile roach
Rutilus rutilus to treated sewage effluent induces dose-dependent and persistent disruption in gonadal duct development. Environ Sci Techol. 2001; 35:462-470.

Tyler CR, Routledge EJ. Natural and anthropogenic environmental oestrogens: the scientific basis for risk assessment, oestrogenic effects in fish in English rivers with evidence of their causation. Pure Appl Chem. 1998; 70:1795-1804.

Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol. 1998; 28:319-361.

Weber LP, Hill RL, Janz DM. Developmental estrogenic exposure in zebrafish (
Danio rerio): II. Histological evaluation of gametogenesis and organ toxicity. Aquat Toxicol. 2003; 63:431-446.