INTRODUCTION
Teleost has a species-specific reproductive cycle and breeding strategy. Their gonadal development and gametogenesis have a strong correlation with the external environment, such as water temperature and the photoperiod in their habitats (Aida, 1973; Caputo et al., 2000). The changes in these environmental factors affect the spawning period (De Vlaming, 1972; Lundquist, 1980; Baek & Lee, 1985; Breitburg, 1987; Caputo et al., 2000). The gobies, mostly small are one of the largest families in the Perciformes. Constituting 10% of the total teleost species, they are in a wide range of area near shores in brackish and freshwater (Nishikawa et al., 1974; Nelson, 1984). Approximately 40 species inhabit the freshwater, brackish water, or shallow water of South Korea (Kim et al., 1987).
The gobies are relatively small, easy to handle, and easily adapt to external environment such as water temperature and salinity. They are found along the shore, vulnerable to contaminants, of the intertidal region and brackish water. Many of the gobiid fish have one or two years total lifespan. Recently, the external environmental changes and contaminants’ influencing the gobiid fish have been studied intensively. Among the gobiid fish, yellowfin goby, Acanthogobius flavimanus and sand goby, Pomatoschistus minutus are being used as environmental indicators (Baek et al., 2004, 2007; Mochida et al., 2004; Ito et al., 2007; Robinson et al., 2007; Saaristo et al., 2009).
The chameleon goby, Tridentiger trigonocephalus, is in the genus Tridentiger of the family Gobiidae. Their habitats extend from China, Japan, Russia, to the northwest region of the USA, and includes the mudflats in the southern and western coastal waters, brackish and freshwater in South Korea (Chung, 1977). Previous studies on the chameleon goby have been investigated about early life history (Kim & Han, 1990), feeding habits (Kim & Noh, 1996), and the tolerance of juvenile exposed to various salinity levels (Kang et al., 2004). However, the characteristics of reproductive biology have not been studied yet. The purpose of this study was to investigate the basic characteristics in reproductive biology of the chameleon goby by histological observation on the gonadal development, changes in gonadosomatic index (GSI), oocyte diameters and fecundity.
MATERIALS AND METHODS
The experimental fish were collected monthly from April 2009 until March 2010 in an eelgrass bed around Dongdae Bay, Namhae, Gyeongsangnamdo, South Korea using a scoop net. During each sample collection, the water temperature was measured using a bar thermometer. Data from the Gyeongnam Meteorological Administration were used for the photoperiod. The samples were measured to the nearest 0.1 cm unit for total length and body length and 0.1 g unit for body weight. The gonad was measured up to the nearest 0.01 g (Fig. 1).
The GSI and the hepatosomatic index (HSI) were calculated by the following formula.
In this formula gonad weight, liver weight, and body weight were measured in grams.
For the histological analysis, gonads were removed, and tissue samples were fixed in Bouin’s solution for 24 hours and then embedded in paraffin. The paraffin-embedded samples were prepared in 5 to 6μm thick sections. The sections were stained with Mayer's hematoxylin-eosin, and observed under a light microscope (BX50, Olympus, Japan). The monthly changes in oocytes’ diameter in the ovaries were measured with a formula of (length+width)/2 and presented in percentage after the microscopic examination on the ovarian tissues using an image analysis device (Moticam Pro205, Motic, Taiwan).
Fecundity (Fc) was measured by a number of isolated oocytes from partial ovary that were not spawned. Fecundity was calculated after isolating oocytes with Gilson solution with a formula shown below; W is gonad weight, ω is partial gonad weight, and ε is weight ratio.
Increase in fecundity with body length, body weight was calculated with a formula of F=a(BL)b, F=a(BW)b.
The reproductive cycle of a female was divided into four stages: growing, maturation, ripe and spawning, and degenerating and recovery. A male also has four stages: growing, maturation, ripe and spermiation, and degenerating and recovery.
RESULTS
The GSI of females began to increase from April, as the water temperature and the photoperiod increased. The GSI was at the highest value (16.31±2.16) in May and 11.69±2.56 in June (Fig. 2-B). The GSI began to decrease drastically from July as the photoperiod decreased and was at the lowest value (0.34±0.03) in August. This was stabilized between 0.45 and 1.61 from September until next the following March, including January when females were not collected. The GSI of males represented a similar tendency, showing a gradual increase from April. It was 0.61±0.08 in May, 0.75±0.07 in June, and showed the highest value in July at 1.17±0.35. The GSI showed a drastic decrease afterwards and was at the lowest value in August at 0.10±0.04 and then stabilized between 0.10 and 0.43 until the next March.
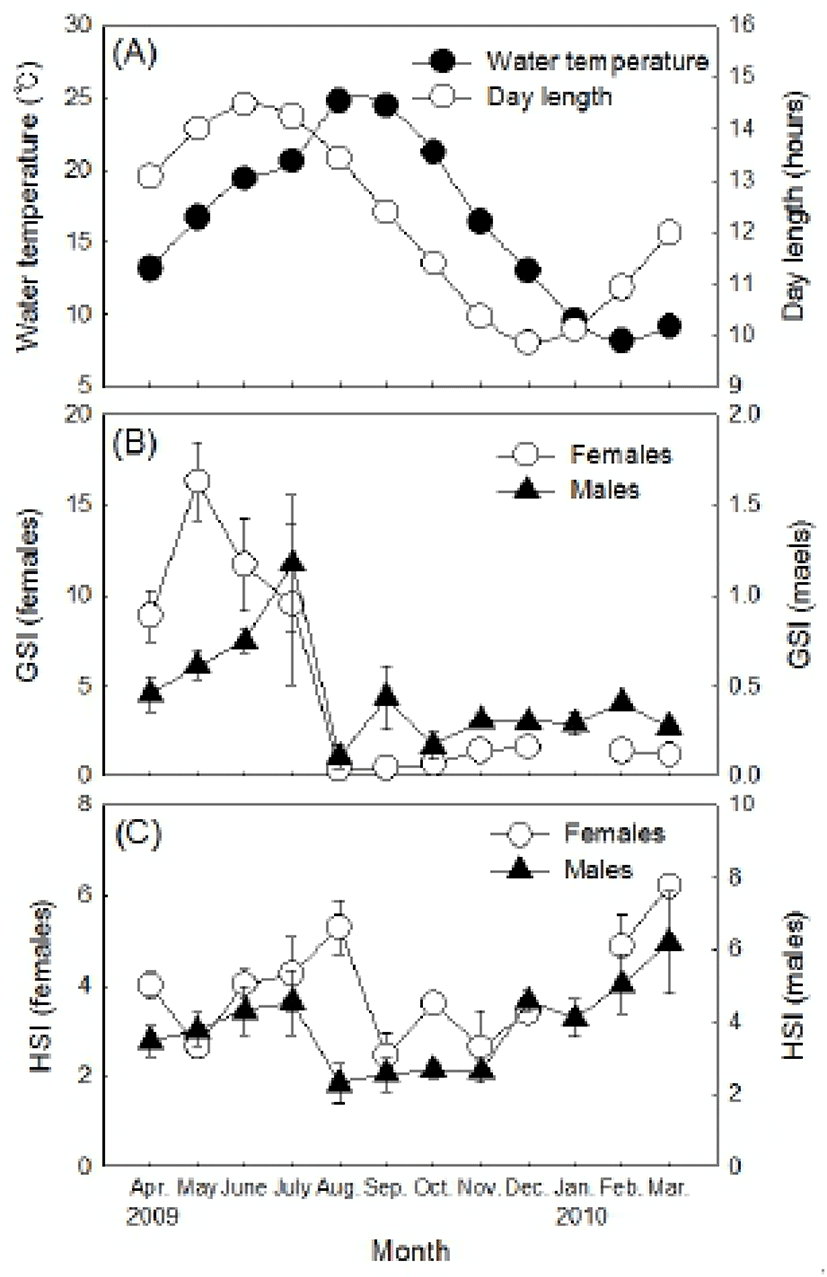
The HSI of females tended to increase gradually from May and was at the highest value (5.31±0.44) in August. Afterwards, it decreased drastically and then stabilized between 2.44 and 3.60 from September until November (Fig. 2-C). The HSI began to increase again from November, showing one sample was relatively higher at 6.21. The males showed a relatively higher HSI between April and July. The HSI, however, decreased drastically afterwards showing the lowest value (2.30±0.39) in August. Then it stabilized between 2.55 and 2.69 until November. Afterwards, the HSI increased and was between 4.10 and 6.20 until the next March.
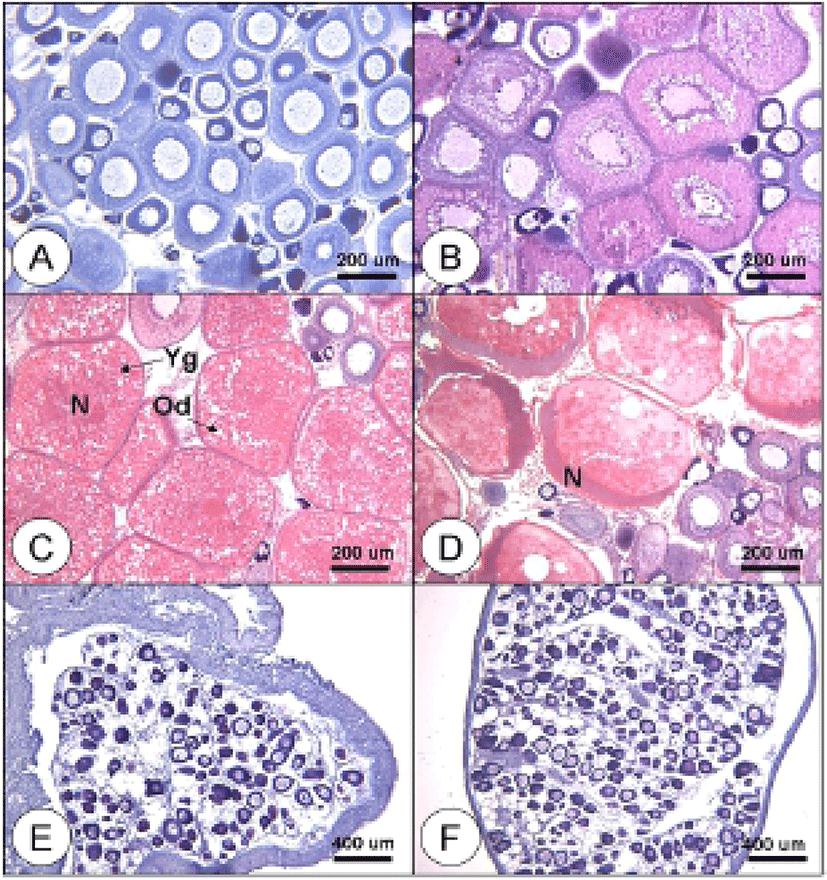
In the growing stage, perinucleolus oocytes less than 100 μm in diameter were observed in the ovaries with the cytoplasm and phosphorus following a positive reaction with hematoxylin. They were also enclosed with follicles measured about 200 μm in diameter (Fig. 3-A). Some oocytes, measured 200 to 300 μm in diameter, developed faster into the oil-droplet stage, showing oil droplets in the inner part of the cytoplasm. Then follicle cells were gradually developed (Fig. 3-B). In the maturing stage, yolk granules and oil droplets became more eosin-basophilic and accumulated in the cytoplasm. The oocytes increased and the diameter ranged from 400 to 450 μm (Fig. 3-C). In the ripe and spawning stage, the oocytes reached 550 μm in diameter. The yolk granules became partially homogenous and oil droplets intermingled with them. Also, the nucleus migrated to the animal pole in this stage (Fig. 3-D). During the degenerating stage, ovarian membrane layer appeared thicker. Basophilic chromatin nucleolus oocytes and peri-nucleolus oocytes were observed in this stage (Fig. 3-E). However, during the recovery stage, the ovarian membrane layer thinned again and the ovary vesicles rearranged. Also, many oocytes less than 100 μm in diameter in the early developmental stage were distributed around epithelium of ovarian vesicles (Fig. 3-F).
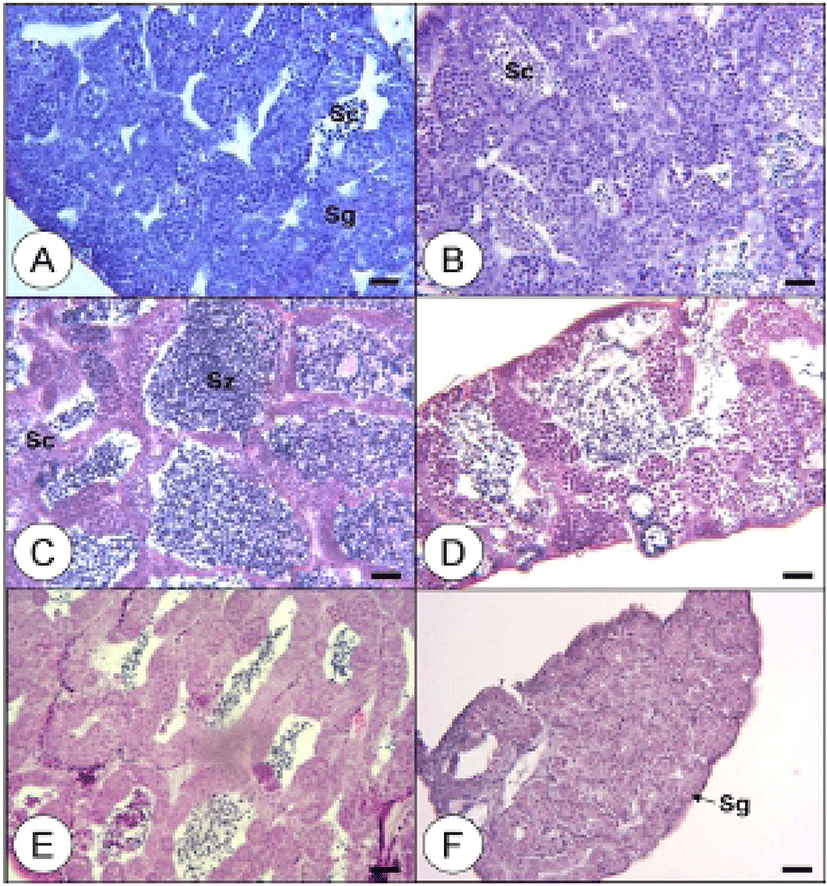
Testes during the growing stage were composed of spermatogonia dividing meiotically into spermatocytes and developing on the epithelium of testicular lobules, which showed a group of spermatocytes (Fig. 4-A, B). In the maturation stage, a large portion of spermatids and a smaller portion of spermatocytes formed in the testicular lobules, demonstrating a positive reaction to hematoxylin by undergoing subdivision and maturation. A group of spermatozoa was produced as well (Fig. 4-C). During the ripe and spermiation stage, testicular lobules expanded and filled with sperm. Some of the matured males released sperm during this stage (Fig. 4-D). In the degenerating stage, a few spermatozoa still remained and spermatogonia were on the epithelium of testicular lobules (Fig. 4-E). During the recovery stage, spermatocytes were distributed, as the epithelium of testicular lobules rearranged its position. The remained spermatozoa degenerated or were absorbed (Fig. 4-F).
The histological observation on the gonadal development and its characteristics divided the reproductive cycle of the chameleon gobies into several stages: the growing stage (from November until March), the maturing stage (April and May), the ripe and spawning stage (June and July), degenerating, and the recovery stage (from August to October) for females and the growing stage (from November until March), the maturation stage (from April to June), the ripe and spermiation stage (July and August), and the degenerating and recovery stage (September and October) for males (Fig. 5).
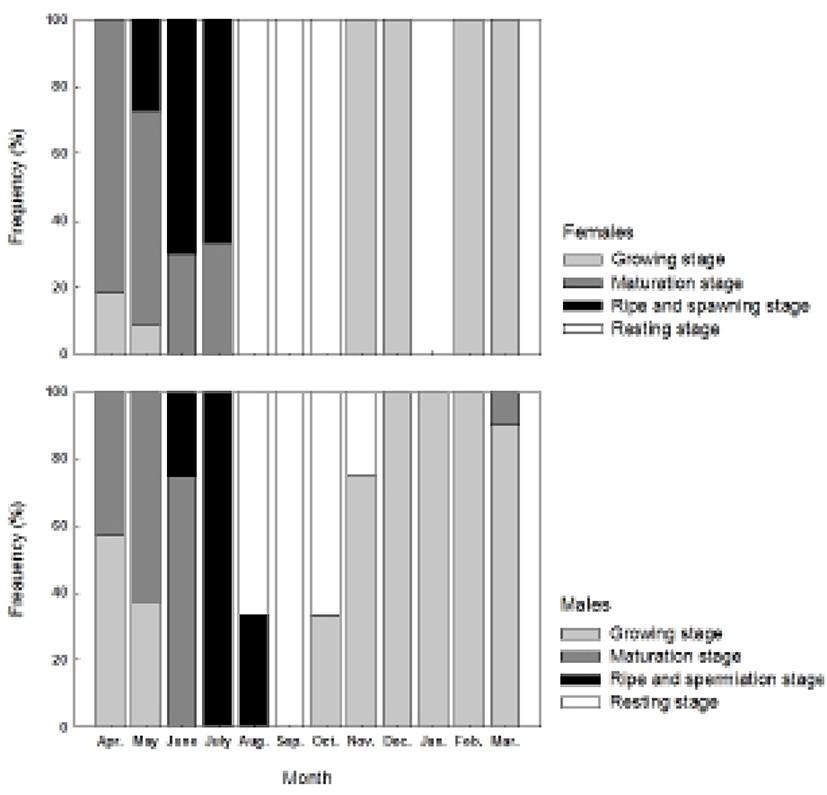
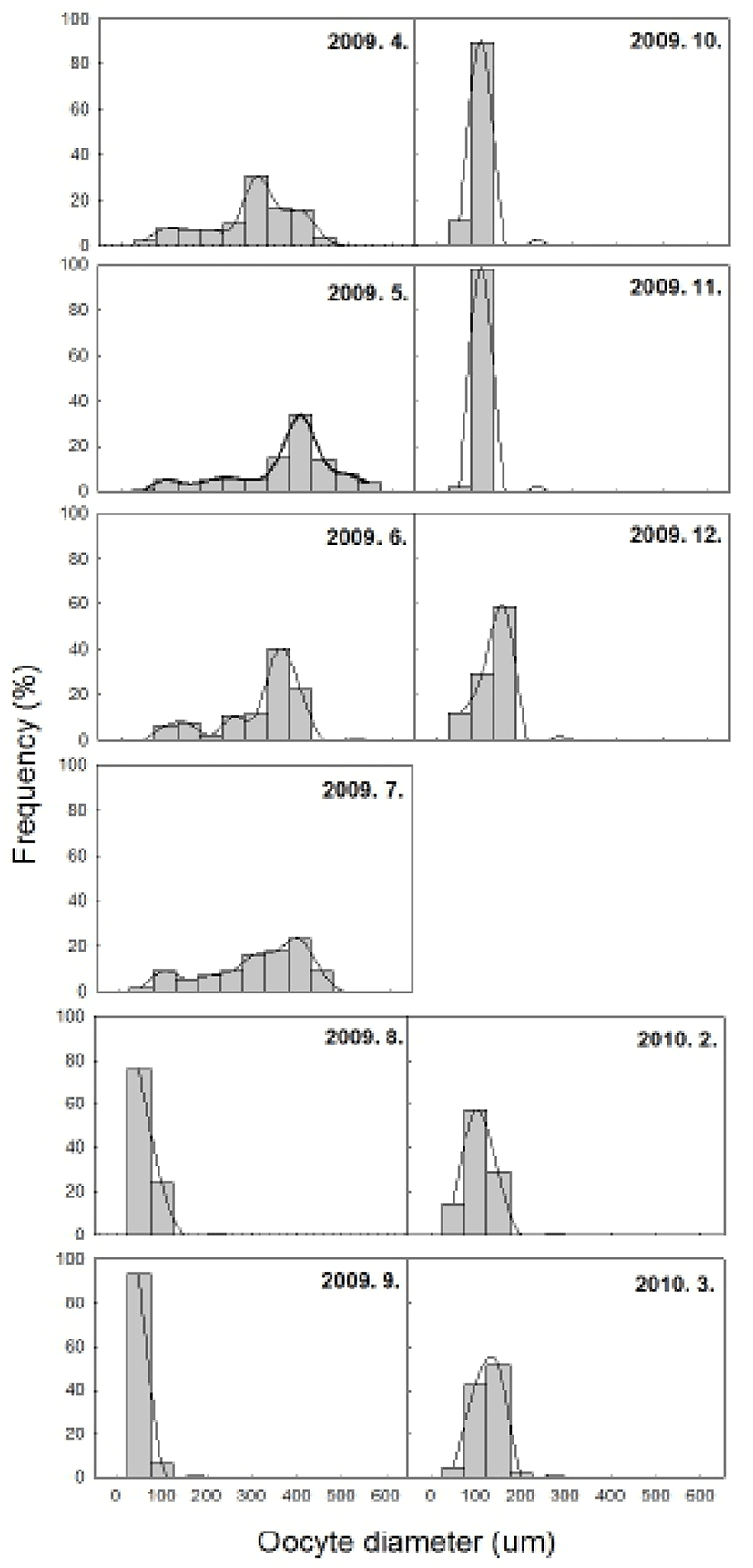
The histological samples from each ovarian developmental stage were observed to measure the changes in oocytes’ diameter (Fig. 6). The perinucleolus oocytes less than 100 μm in diameter were noted all year around. From April until July, the oocytes measuring from 200 up to 600 μm in diameter were observed as they grew and matured. After July, no oocytes larger than 100 μm in diameter appeared until March.
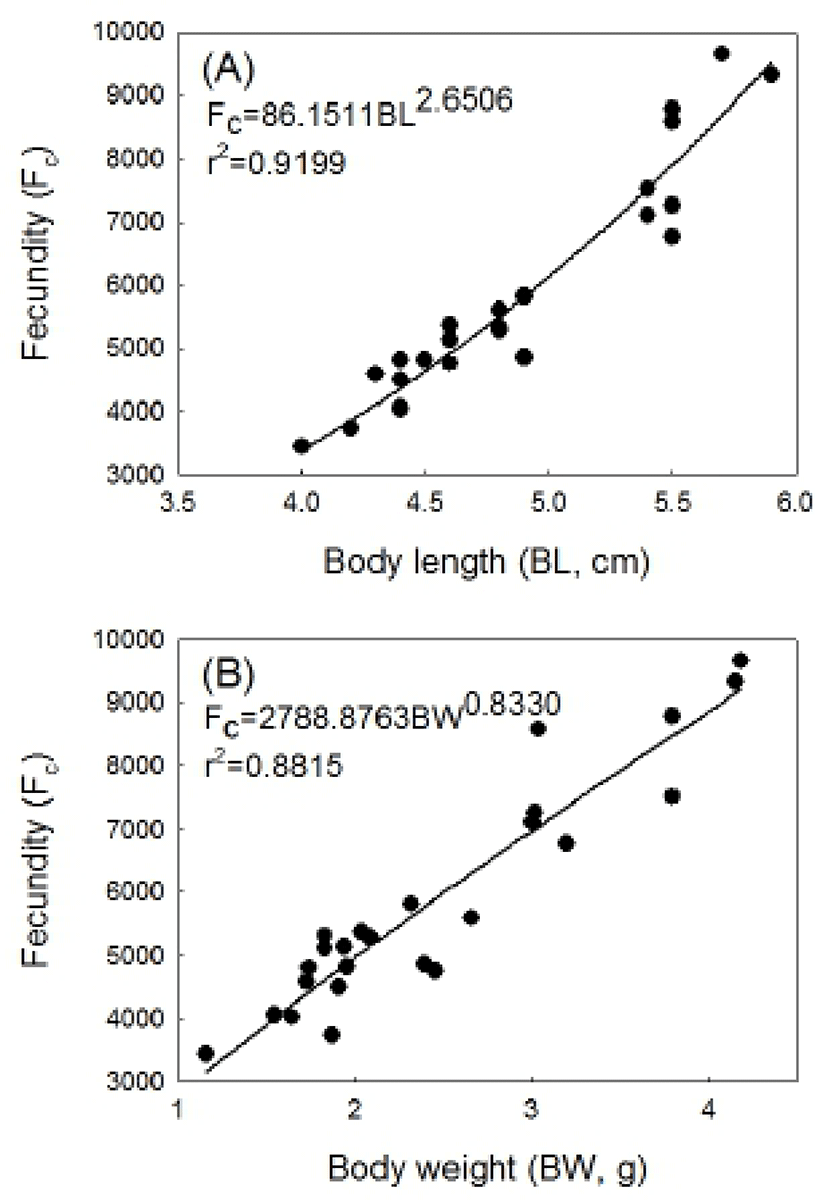
Fecundity from the mature females was measured between 3,448 and 9,654 (Fig. 7). The samples with body length of 4.0 to 5.0 were rated 4,781 in fecundity and the ones with the body length of 5.2 to 5.9 cm 8,124, which indicates fecundity increases positively correlated to body length increases. The relation of the body length to fecundity is modeled by the formula Fc=86.1511BL2.6506 (r2=0.9199) (Fig. 7-A). The samples with body weight of 1.16 to 1.99g had an average fecundity 4,507 and the ones with the body weight of 3.00 to 4.19g 8,124. This indicates fecundity is positively correlated to body weight increases. The relation of the body weight to fecundity is modeled by Fc=86.1511BL2.6506 (r2=0.9199) (Fig. 7-B).
DISCUSSION
The reproductive cycle and spawning season are known to be regulated by periodical changes of water temperature and photoperiod. The spawning patterns of fish are divided into spring-spawning, summer-spawning, and winter-spawning according to the seasonal change (Heath, 1987; Jobling, 1995). Results from this study show the female chameleon gobies inhabiting Namhae-gun, Gyeongsangnam, South Korea had it’s the highest GSI in May, then decreased from June and had it’s the lowest value in August. The GSI of males was at it’s the highest value in July and the lowest value in August. These results indicate the chameleon gobies start spawning from May and are considered as spring-summer spawners and peak spawning season is between June and July. The goby species with a similar spawning season are the dusky tripletooth goby, Tridentiger obscurus, naked-headed goby, Favonigobius gymnauchen peaking in June and July, striped goby, Acentrogobius pflaumi in May and June, and longchin goby, Chasmichthys dolichognathus between April and July. As the increased water temperature and long photo-period are the main factors controlling maturation and spawning, the high water temperature in summer induces gonadal degradation in longchin gobies and trident gobies and inhibits maturation (Baek et al., 1985; Kaneko & Hanyu, 1985; Lee et al., 2000; Baek et al., 2004; Jin et al., 2006). This study shows the changes of GSI related to the environmental factors in the chameleon gobies’ habitat showing the GSI of the females increased from April, when the long photoperiod occurred. Then the GSI was at the highest in May and dropped rapidly in August, when it had the highest water temperature of the year. As for the males, the GSI was at the highest value in July and then dropped in August showing the similar changes with the females. These results suggest that the gonadal development activates as the water temperature increases by long photoperiod, and high water temperature in summer season induce gonadal degeneration. However, further studies on the relation of controlled water temperature and photoperiod to facilitate gonadal development, spawning or degradation should be conducted to clarify these interactions.
The HSI of the chameleon gobies did not indicate any significant relation to the GSI changes in females or males. However, the changes in HSI during the spawning season are related to the GSI changes. The female GSI reached it’s the highest value in May, but the HSI reached it’s the highest value in August. Also, the GSI began to decrease constantly between May and August, but the HSI increased during the same period. Taken together, these results suggest that the HSI increases during winter season as vitellogenin (VTG) and nutrients synthesize actively in the liver, VTG is transferred to the ovaries in April and May then the HSI decreases, but it increases again after vitellogenesis.
The changes in HSI have a close relation with reproductive factors such as nutrition accumulation and consumption, feeding habit, and vitellogenesis (Aida et al., 1973). The HSI and GSI changes were contrary to each other for the yellow croaker, Larimichthys polyactis (Kang et al., 2006), the starry flounder, Platichthys stellatus (Lim et al., 2007), and the gluttonous goby, Chasmichthys gulosus (Kim et al., 2004). However, the GSI of the striped goby, Acentrogobius pflaumi (Baeck et al., 2004), the greenling, Hexagrammos otakii (Lee et al., 2000), and the sea bass, Lateolabrax japonicus (Kang et al., 2001) have a proportional correlation with the HSI since VTG accumulates in a liver. This is thought because the period of VTG and nutrients production, storage and transfer to the gonads from the liver differs among the species. The male chameleon gobies have a contrary tendency to the females, showing both GSI and HSI constantly increasing from April until July and then decreasing to its lowest value in August. The reason for the HSI and GSI of males during spawning season increase is due to the nutrition accumulation to store energy for the reproduction and to protect the fertilized eggs (Kim & Han, 1990).
The ovaries of matured chameleon gobies were distributed mostly with mature oocytes 400 to 550 μm in diameter. The largest oocyte in diameter was 550 μm, and the yolk granules and oil droplets became homogenous, which occurred only in samples collected in May. Until July, the oocytes larger than 400 μm in diameter were found and the most of oocytes were immature with 100 μm in diameter since August. Also, the histological examination showed the ovaries from May until July had no spawning marks. In addition, monthly collected samples of both males and females showed a similar range in body length with the ones collected during the spawning season. Therefore, the chameleon gobies are considered to spawn once in a spawning season and to survive after the spawning. Other gobiid species with a similar spawning season, such as the naked-headed goby, Favonigobius gymnauchen and striped goby, Acentrogobius pflaumi also spawn once during the spawning season (Lee et al., 2000; Baeck et al., 2004). However, gluttonous goby, Chasmichthys gulosus spawn several times between February and April (Kim et al., 2004). The dusky tripletooth goby, Tridentiger obscures (Jin et al., 2006) also spawn several times during the spawning season and the authors hypothesized that most of the spawned fish die shortly since the body length decrease after the spawning season. It is known abbreviate iteroparous types, multiple spawners during one year of spawning, have a shorter life span than a year because multiple spawning cause necrosis of gonad tissues and starvation for fertilized egg protection (Caputo et al., 2000). However, bluespot gobies, Pseudogobius olorum are reported to survive until the next spawning season if they do not spawn that year (Gill et al., 1996).
The results from fecundity examination show chameleon gobies produce 3,448~9,654 eggs. The samples with a body length of 6.8 cm and body weight of 4.19 g showed the largest egg numbers, 9,654. Also, as the body length and body weight increased, the fecundity increased. The fecundity of the gobies varied depending on the species, but mostly it increased as the body length and body weight increased (Song & Baek, 2005). It was reported that trident gobies, Tridentiger obscurus produced 1,214~ 13,892 eggs (Jin et al., 2006), 151~2,209 eggs in freshwater goby, Rhinogobius brunneus (Song & Baek, 2005), and 3,613~9,773 eggs in striped goby, Acentrogobius pflaumi (Baeck et al., 2004). In conclusion, the chameleon gobies have annual reproductive cycle; the GSI for both males and females increase from April, when it is long photo-period. The peak spawning occurs in June and July, and then spawning terminates in August. The histological observation of the gonad indicates that the chameleon gobies are considered to spawn once in a spawning season and show an increased fecundity when the body length and body weight increase.