INTRODUCTION
The Ussurian bullhead, Leiocassis ussuriensis, is a fish species of the family Bagridae. It occurs in the Daedong River, Amnok River, Imjin River, Han River, and Geum River of Korea, and has also recently been found in the Nakdong River of Korea (Kim & Park, 2002). It is a nocturnal fish that lives in rivers with slow currents, on river bottoms composed of mud or sand. With its chewy flesh and excellent umami taste, it is an essential ingredient of the local spicy freshwater fish stew, together with the Korean bullhead, Pseudobagrus fulvidraco (Lim et al., 2012).
The Korean bullhead, which also belongs to the family Bagridae (Park & Lee, 1996), is found throughout Asia, including Korea, and commonly inhabits the West Sea and South Sea flows into the midstream and downstream of the rivers in Korea (Park & Lee, 1996; Kim & Park, 2002). The Korean bullhead has no scales on its body and its head is flat. It is a predominantly carnivorous fish, feeding at night, so its habit is nocturnal (Lee, 1993). The Korean bullhead is considered to have a spectacular taste and is widely used as the main ingredient in spicy freshwater fish stews, as are catfish and carp, and the demand for this fish is therefore increasing (Lee, 1993).
The visual function of fish larvae is important for their feeding, positioning, collective behavior, and escape from predators (Rodriguez & Gisbert, 2001; Park et al., 2006a, 2006b). The retinal structure of the Teleostei does not differ from that of other vertebrates, but the number of teleost species and their various habitats, activities, and life cycles make it difficult to define a general retinal structure for the Teleostei (Wagner, 1990). The behavioral and environmental factors affecting different fish are important to their visual ecology and to any study of their visual structures and function (Walls, 1942; Lythgoe, 1979).
There has been much research into yolk absorption and early growth in the rainbow trout, Oncorhynchus mykiss (Park et al., 1996) and chum salmon, O. keta (Zhang et al., 1995), and into the eye development of the dotted gizzard shad, Konosirus punctatus (Park et al., 2006a) and the dark-banded rockfish, Sebastes inermis (Park et al., 2012), and morphometric and hitological changes in the cyprinid loach, Misgurnus anguillicaudatus (Han et al., 2013). Generally, the development of an internal organ, such as the kidney or midgut epithelium, proceeds throughout the larval stage (Blaxter, 1988). However, there have been insufficient studies of the Ussurian bullhead and Korean bullhead. Also, the Ussurian bullhead and Korean bullhead have similar habitats and spawning seasons. Comparative histological analysis two species is semantic. Therefore, the aim of this study was to observe the histological development of these two species, which should provide important indices for basic research into the internal changes that occur in Bagridae larvae.
MATERIALS AND METHODS
In this study, we examined juvenile specimens of the Ussurian bullhead, Leiocassis ussuriensis, and the Korean bullhead, Pseudobagrus fulvidraco. On June 28, 2012, we fertilized eggs of the two species, which were reared and bred at the Fisheries Genetics and Breeding Sciences Laboratory, Korea Maritime and Ocean University, Busan, Republic of Korea. The larvae were fed three times a day (08:00, 12:00, and 17:00 h) exclusively with Artemia metanauplii (Salt Creek Inc., Salt Lake City, UT, USA). The larvae were reared in 10 tanks (100 L) that included a circulation pump, an aeration system, and a temperature control system. Dissolved oxygen levels were maintained with an air pump, and the water temperature was maintained at 26±0.5°C.
We extracted the eye, kidney, and midgut epithelium needed for histological observation from each species at 50 DPH. Each sample was fixed in Bouin’s solution for 24 h and then washed in flowing water. The samples were then processed in decalcification solution for 24 h, washed again, and dehydrated through a graded series of alcohol (70%, 80%, 90%, and 100% alcohol for 1 h each). The samples were then cleared with xylene and impregnated with soft paraffin and hard paraffin. After impregnation, the samples were embedded, trimmed, and cut. It stained with hematoxylin-eosin. The samples were then mounted with Canadian balsam, examined with an optical microscope, and photographed with an optical microscope camera (AxioCam MR, Carl Zeiss, Germany).
Using an eyepiece micrometer under an optical microscope (Carl Zeiss, Germany), samples of each species, ranging from just hatched to 50 DPH, were randomly selected and the retinal thickness measured. We calculated the proportional thicknesses of the epithelial layer (EL), rod and cone layer (RCL), outer limiting membrane layer (OLM), inner nuclear layer (INL), inner plexiform layer (IPL) and ganglion cell layer (GCL) of the retinas, using the method of Park et al. (2006b), in just-hatched, 10 DPH, 30 DPH, and 50 DPH specimens of both species. To determine the development of the kidney and midgut epithelium, a biological microscope was used to measure the areas and volumes of the cells and nuclei using the following formulae: surface area=1/4×abπ, and volume=4/3×π(a/2)×(b/2)2, where a=the major axis of the cell or nucleus; b=the minor axis of the cell or nucleus (Park & Kim, 2000). We also photographed the eye structure and the developmental stages of the kidney and midgut epithelium in each just-hatched and 50 DPH specimen using the Axioskop 4.1 image analysis software (Carl Zeiss, Germany).
The experiment was performed in triplicate and the results are reported as means±SD (n=30), unless otherwise stated. The data were analyzed with one-way ANOVA using the SPSS statistical package (SPSS 9.0, SPSS Inc., Chicago, IL, USA). Means were compared with Duncan’s multiple range test, and were considered significantly different at p<0.05.
RESULTS
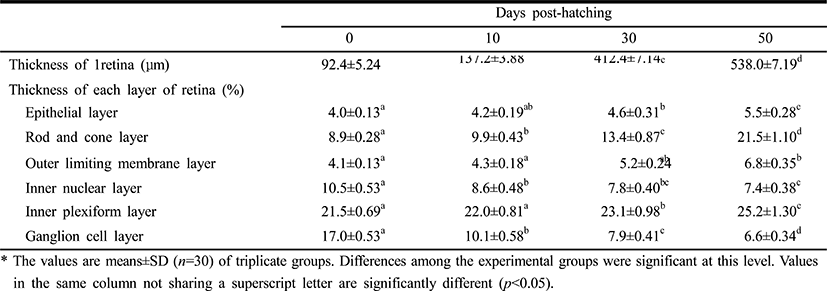
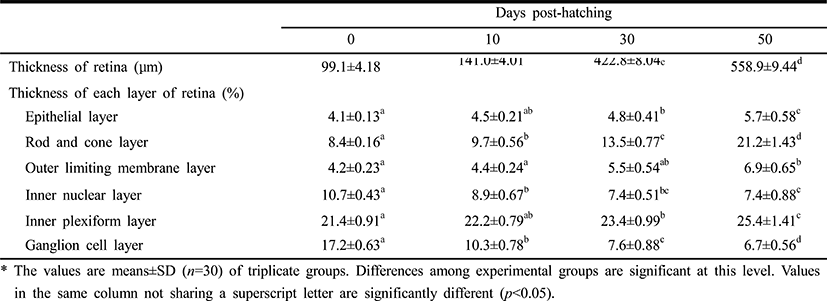
Table 1 lists the component layers of the retina in the Ussurian bullhead, Leiocassis ussuriensis, from just-hatched to 50 DPH. The thickness of the retina increased continuously from 92.4 μm to 538.0 μm in 50 DPH (p<0.05). The retina consisted of the EL, RCL,OLM, INL, IPL, GCL,and lens (L). The thickness of the EL, RCL, OLM, and IPL increased, but the INL and GCL decreased in thickness in 50 DPH (p<0.05). The thickness of the RCL increased most rapidly and the thickness of the GCL decreased most dramatically of all the component layers in this period (p<0.05). In case of Korean bullhead, Pseudobagrus fulvidraco, the component layers of the retina from just-hatched to 50 DPH shown at Table 2. The retinal thickness increased continuously in common with Ussurian bullhead from 99.1 μm to 558.9 μm during 50 DPH (p<0.05). Also, the Korean bullhead retina consisted such as EL, RCL, OLM, INL, IPL GCL, and L. Likewise, the thickness of the EL, RCL, OLM and IPL increased, the thickness of the INL and GCL decreased during 50 DPH (p<0.05). The thickness of the RCL increased most rapidly and that of the GCL decreased most dramatically of all the component layers in this period (p<0.05). Thus, both species showed similar trends in retinal development, with the INL and GCL decreasing and the RCL increasing rapidly. The increase in retinal thickness was faster in the Korean bullhead than in the Ussurian bullhead, but the relative proportions of the retinal layers did not differ significantly between the two species. Although the retinal growth of the Korean bullhead was faster, the relative proportions of the retinal layers did not change during early development.
|
|
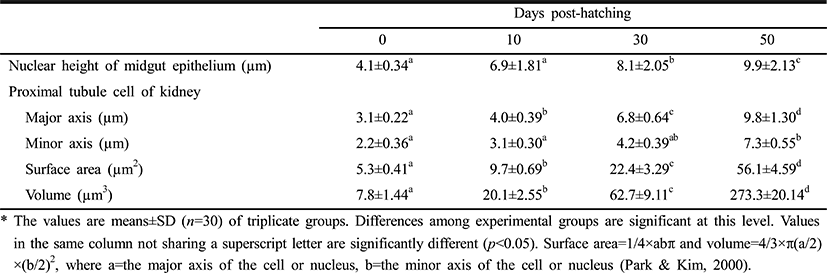
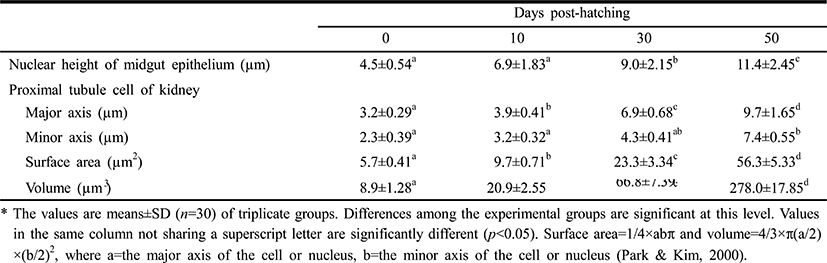
Table 3 lists the nuclear heights of the midgut epithelium cells and the cellular dimensions of kidney in the proximal renal tubules of the Ussurian bullhead at just-hatched, 10 DPH, 30 DPH, and 50 DPH. The nuclear height in the midgut epithelium increased continuously from just-hatched to 50 DPH (p<0.05). The major axes and minor axes of the proximal tubule cells in the kidney increased with time, and the minor axes were shorter than the major axes throughout this period (p<0.05). The surface areas and volumes of the renal proximal tubule cells increased dramatically with time (p<0.05). Table 4 lists the nuclear heights in the midgut epithelium and the dimensions of the cells in the renal proximal tubule of the Korean bullhead at just-hatched, 10 DPH, 30 DPH, and 50 DPH. The nuclear height in the midgut epithelium increased continuously from just-hatched to 50 DPH (p<0.05). The major axes and minor axes of the proximal tubule cells in the kidney increased with time, and the minor axes were always shorter than the major axes (p<0.05). The surface areas and volumes of the renal proximal tubule cells increased dramatically with time (p<0.05). Thus, the two species showed similar trends in these parameters, with increases in nuclear height and cell size as development progressed.
|
|
Fig. 1 shows the histological changes in the eye of the Ussurian bullhead from just-hatched to 50 DPH. Fig. 1A shows the just-hatched eye, which has a much looser layer structure than the eye at 50 DPH, shown in Fig. 1B. In particular, the RCL and OLM are loose immediately after hatching, but become densely concentrated by 50 DPH. Fig. 2 shows the histological changes in the eye of the Korean bullhead from just-hatched to 50 DPH. In Fig. 2A, the just-hatched eye has a much looser layer structure than that of the eye at 50 DPH, shown in Fig. 2B. In particular, the RCL and OLM are loose just after hatching, but are densely concentrated at 50 DPH. Both species showed gradually increasing retinal density with development.
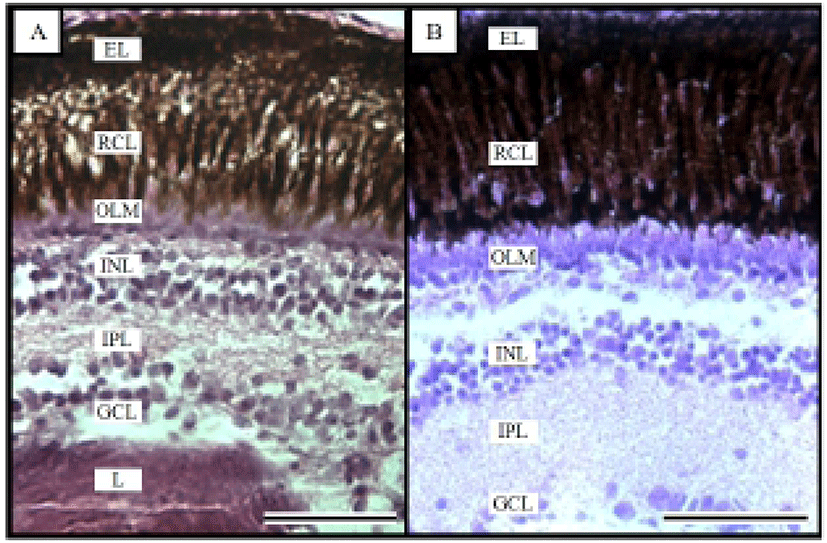
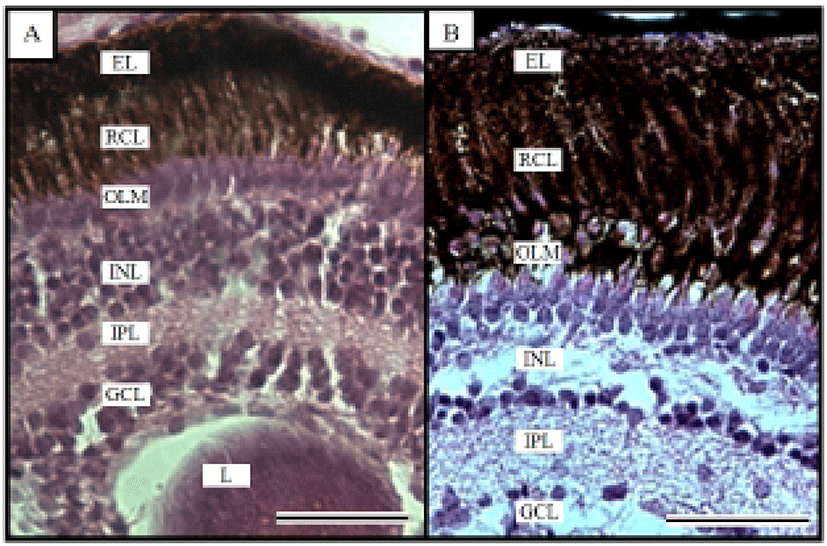
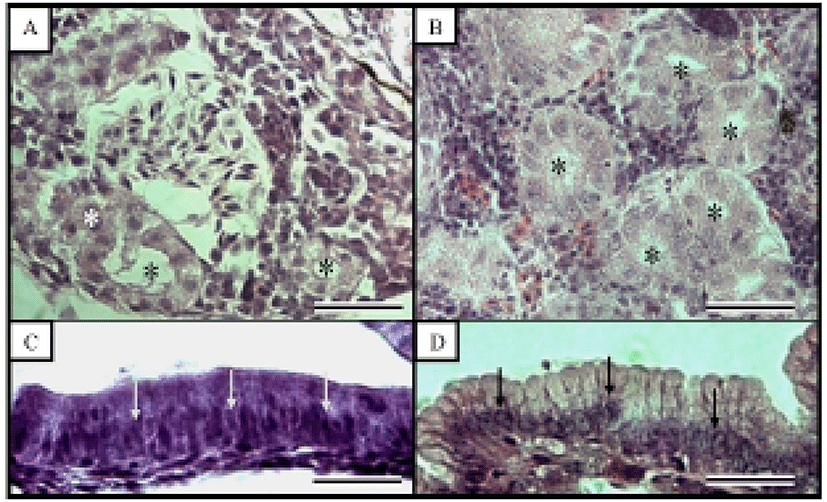
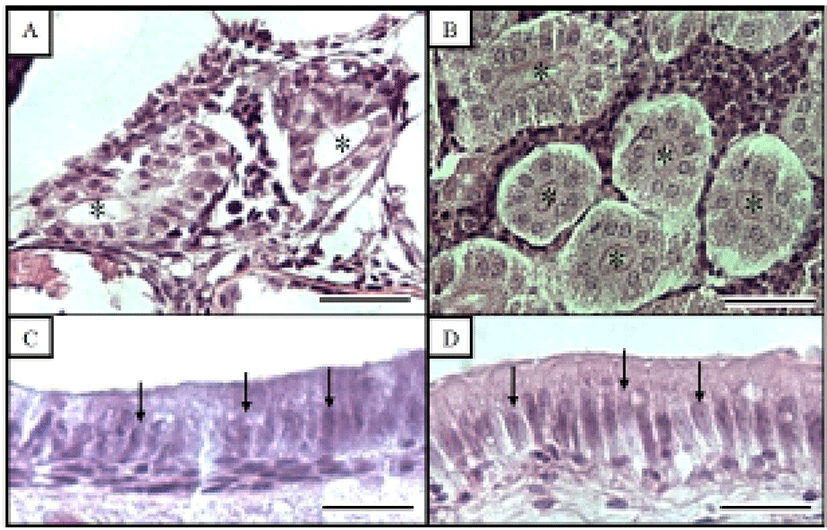
Fig. 3 shows the histological changes in the kidney and midgut epithelium of the Ussurian bullhead from just-hatched to 50 DPH. The kidney consists of many proximal tubules, which appear as hollow ovals or circles. Fig. 3B shows the kidney at 50 DPH, which has more and larger proximal tubules than the just-hatched kidney in Fig. 1A. Of the three tissues examined in this study, the greatest differences were seen in the midgut epithelium during the 50 days of the experiment. The midgut epithelium at just-hatched (Fig. 3C) is tightly stretched but that at 50 DPH (Fig. 3D) is wrinkled and folded, and the density of the tissues has increased (Fig. 3D). Fig. 4 shows the histological changes in the kidney and midgut epithelium of the Korean bullhead from just-hatched to 50 DPH. The kidney consists of many proximal tubules, which appear as hollow ovals or circles. Fig. 4B shows the kidney at 50 DPH, which has more and larger proximal tubules than the just-hatched kidney (Fig. 2A). Of the three tissues examined in this study, the greatest changes were seen in the midgut epithelium during the 50 days of the experiment. The midgut epithelium at just-hatched (Fig. 4C) is tightly stretched, but that at 50 DPH (Fig. 4D) is wrinkled and folded, and the density of the tissue has increased (Fig. 4D). A comparison of the histological changes in the kidneys and midgut epithelia of the two species showed increases in tissue density and histological changes with time.
DISCUSSION
In the teleostei, the eye usually continues to grow into adulthood, but the optical characteristics of the eye components stabilize in the early days of development, and the eyes of the Ussurian bullhead, Leiocassis ussuriensis, and the Korean bullhead, Pseudobagrus fulvidraco, were completely formed just after hatching. Their retinas are composed of six layers, being EL, GCL, INL, IPL, OLM, and RCL (Figs. 1 and 2), and each layer showed characteristics similar to those of other cyprinid fish (Takashi, 1982). In this study, trends of the retina layer changed were similar in the two species, which is consistent with the observations of Park & Kim (2000). Similar research has been conducted in the dark-banded rockfish, Sebastes intermis, in which the RCL did not change significantly during development, whereas there was a significant change in the OLM (Park et al., 2012), and similar result in the cyprinid loach, Misgurnus anguillicaudatus (Han et al., 2013). This result is contrary to our observations in the Ussurian bullhead and Korean bullhead. Based on the results of this study and the intrinsic functions of the retinal components, we infer that the rates of change in the retinal components of fish are related to their physiology and ecology in the early days of their development.
The early development of internal organs does not differ in most Teleostei (Blaxter, 1988). Generally, there are two types of renal development, with the kidney forming either before or after hatching. Proximal tubules exist in kidney and using to indicator for histological comparison, proximal tubule cells measured such as major axes and minor axes (Han et al., 2013). Most aspects of the kidney tissues were similar in the two species examined here, although the Ussurian bullhead had more and larger proximal tubules than the Korean bullhead (Figs. 3 and 4). In this study, the kidneys of the Ussurian and Korean bullheads were already formed when the fish hatched and were fully developed by 50 DPH. This tendency has also been observed in the dentex, Dentex dentex and cyprinid loach, which has a fully formed kidney at hatching, which continues to grow thereafter (Santamaria et al., 2004; Han et al., 2013).
Nuclei height of midgut intestine epithelium was using the indicator for histological research, hence used to nuclei height of midgut intestine epithelium for comparative two species in this experiment (Theilacker, 1978; Park, 2006, Han et al., 2013). Most aspects nuclear height of the midgut epithelial were also similar in the two species, although the nuclear height was flatter in the Korean bullhead (Tables 3 and 4; Figs. 3 and 4). It has been reported that the absorptive function of the alimentary canal in fish begins to function either before or after the absorption of the yolk sac. In the juvenile Coregonus fera, the yolk sac is completely absorbed by 11 DPH, and during this period the absorptive activity of the midgut epithelium begins to function (Loewe & Eckmann, 1988). In the juvenile summer flounder, Paralichthys dentatus, the yolk sac is completely absorbed by 3 DPH and the absorptive activity of the midgut epithelium begins to function at 4 DPH (Bisbal & Bengtson, 1995). However, in the juvenile pike eel, Muraenesox cinereus, the absorptive function of the midgut epithelium begins at 3 DPH, before the complete absorption of the yolk sac. Although the mucosal fold of the midgut has not formed at this time, the microvillous structures are detectable in the midgut with optical microscopy, and the active absorption of horseradish peroxidase is detectable in these cells, so liquefactive nutriments can be absorbed at this time (Otake et al., 1995). As soon as the Ussurian bullhead and Korean bullhead hatched, their midguts were already present and grew gradually to 50 DPH. This tendency has also been observed in the cyprinid loach (Han et al., 2013). Because the yolk sac was only completely absorbed after 14 DPH in both species, the absorptive function of the midgut must begin before the yolk sac is completely absorbed in these species, as in the pike eel or cyprinid loach.
As mentioned above, there has been little research into the early growth of the Ussurian bullhead and Korean bullhead, which share the same family and similar habitats. In this study, we examined the histological development of both species from 1 DPH to 50 DPH. Our results may provide useful information for the successful rearing of the Ussurian bullhead and Korean bullhead.