INTRODUCTION
Orchidectomy (also spelled orchiectomy) is a surgical procedure to remove testes and thought to be the oldest method of castration. In urological field, this procedure extensively used if testis cancer and prostate cancer were suspected (Moul, 2007; Damber, 2005). More recently, chemical castration which eliminates androgen by using hormone analogs has been widely used in the researches and medical remedy (Damber, 2005). Both surgical and chemical castration methods, however, have critical disadvantages. Though the surgical procedure is effective, infection or bleeding can bring a serious condition and it is time-consuming (Jana & Samanta, 2007). On the other hand, hormone-based chemical castration is expensive and not cost-effective, frequently exerting the drug resistance in long-term therapy (Oefelein & Cornum, 2000).
Several laboratories have developed nonsurgical sterilization methods, mostly using single intratesticular injection of simple salt solutions such as calcium chloride (CaCl2) and hypertonic saline (Jana & Samanta, 2006, 2007; Emir et al., 2008; Kwak et al., 2010). In particular, chemoablation of testes with hypertonic saline solution was found effective and were guaranteed minimal safety not only in the rats but in the human patients (Emir et al., 2011). To ensure the successful development and application in medical and research fields, however, these chemo-sterilization methods need to be more precisely studied in patho-physiological aspects.
In the present study, we further verify the efficacy of the hypertonic saline injection method using protein and DNA gel electrophoretic analyses.
MATERIALS & METHODS
Male Sprague-Dawley rats (5 months old) were obtained from DBL (Chungcheongbuk-do, Korea) and acclimated 1 week in our animal facility under conditions of 12-h light/dark cycle (lights on at 07:00 h) and constant temperature of 22±1°C. Animal care and experimental procedures were approved by the Institutional Animal care and the use committee at the Sangmyung University (R-1301) in accordance with guidelines established by the Korea Food and Drug Administration.
Rats were divided into three groups including intact group (intact), orchidectomy group (ORX) and saline injection group (SAL). Sterilized 20% saline was directly injected into the animals (750 μl per testis) using insulin syringe. Bilateral orchidectomy was performed at the same day of the saline injection. All rats were sacrificed at 4 weeks after the saline injection. The reproductive tissues(testes, epididymis, seminal vesicles and prostates) were collected and used for DNA and protein pattern analyses.
Total DNAs were extracted using the G-DEX™IIc genomic DNA extraction kit (iNtRON, Korea). Briefly, reproductive tissues were homogenized in cell lysis buffer using homogenizer (IKA, Germany). Homogenate was treated with RNase solution for 30 min at 37°C. 100 μl of protein precipitation (PPT) buffer was added to the homogenate, and the homogenate was vigorously vortexed at high speed for 20 seconds. After vortexing, the homogenate was centrifuged at 12,000 rpm for 5 minutes and transferred the 300 μl of supernatant to a 1.5 ml tube. 300 μl of 100% Isopropanol (Merck, Germany) was added to the sample and the sample was centrifuged at 12,000 rpm for 1 minute. The DNA pellet was dried and washed with 1 ml of 70% ethanol. Finally, 150 μl of DNA rehydration buffer was added to the extracted DNA and the resuspended samples were loaded into 2% agarose gels and separated by electrophoresis.
The tissues were immediately placed in cold RIPA buffer [50 mM Tris (Biopure, Korea; pH 7.4), 150 mM NaCl (Sigma, USA), 1% Triton X-100 (Sigma, USA), 1 mM Phenylmethylsulfonyl fluoride(PMSF)(Sigma, USA)] and homogenized. The homogenate was centrifuged at 13,000 rpm for 20 minutes at 4°C and the supernatant was transferred to a new 1.5 ml tube. Protein quantity of the sample was measured using Bradford quantitative analysis method. After adding of 5X SDS dye, the protein samples were heated for 5 minutes at 90°C and stored at –20°C for further analysis. The protein samples were warmed up to 37°C, loaded into 10% polyacrylamide gels and were separated by electrophoresis for 2 hours at 100V. The total protein amount in each sample was adjusted by staining with Gelcode Coomassie Blue Stain Reagent (BioRad, USA) in order to provide equal loading.
Testes were fixed 4% paraformaldehyde overnight at 4°C for 24 h, and then were serially dehydrated in graded ethanol and xylene. The fixed tissue were dehydrated in ethanol (70%, 80%, 90%, 95%, 100%) and embedded in paraffin block. The tissues blocks were cut at 4-5 μm using microtome (HM350S, MICROM, Germany). Sections were stained with hematoxylin-eosin and observed using a light microscope (BX51, Olympus, Japan).
RESULTS
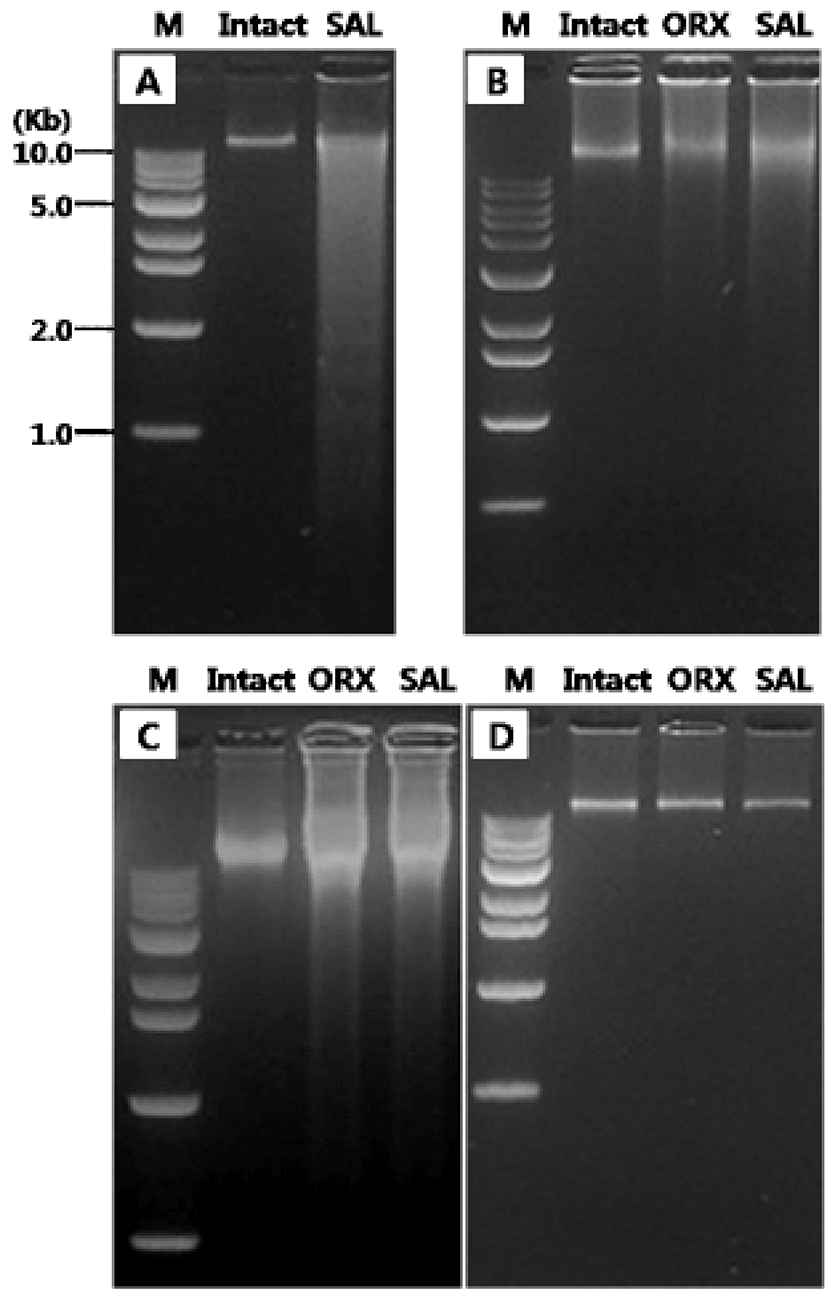
The first experiments were designed to compare the gDNA patterns of reproductive tissues and to elucidate if their pattern was differentially altered by the androgen deprivation methods (Fig. 1). We failed to observe the DNA ladder, a typical sign of apoptotic DNA fragmentation, in all samples (Fig. 1A-D). The gDNA gel electrophoresis of rat testes revealed that there was no breakdown of the gDNA in intact testes while broad range of ethidium bromide gDNA staining in the SAL group testes (Fig. 1A). In epididymal gDNA study, control group shown intact state of gDNA while those from ORX group and SAL group exerted the broken gDNAs which were more moderate than in saline injected testes (Fig. 1B). Similar pattern of gDNAs were found in seminal vesicles (Fig. 1C). However, the gDNAs from all three group shown intact state (Fig. 1D).
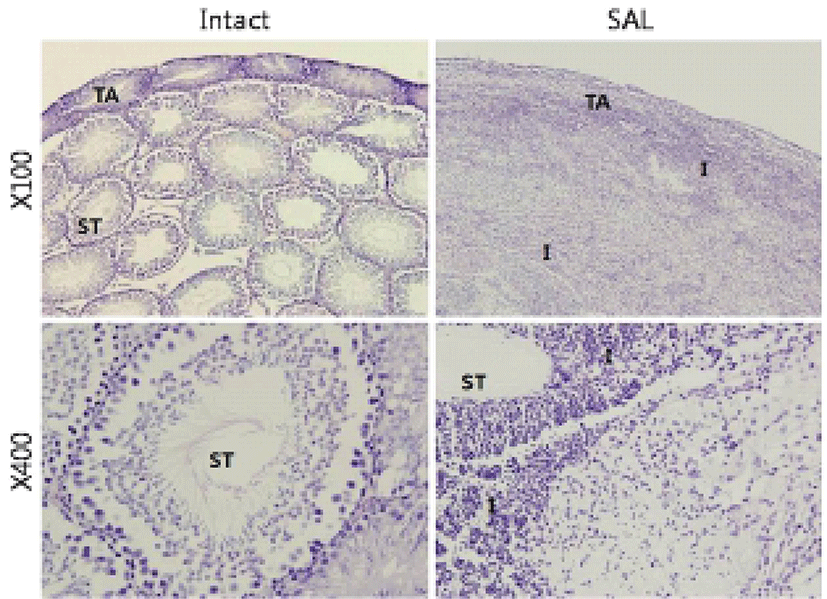
The second experiments were patho-histological approach to find whether the tissue gDNA pattern changes induced by hypertonic saline injection are correlated with degeneration testicular microstructure. While testes of the intact group were observed normal seminiferous tubules (Fig. 2), almost all testicular tissue underwent severe destruction with massive infiltration of immune cells in SAL group (Fig. 2).
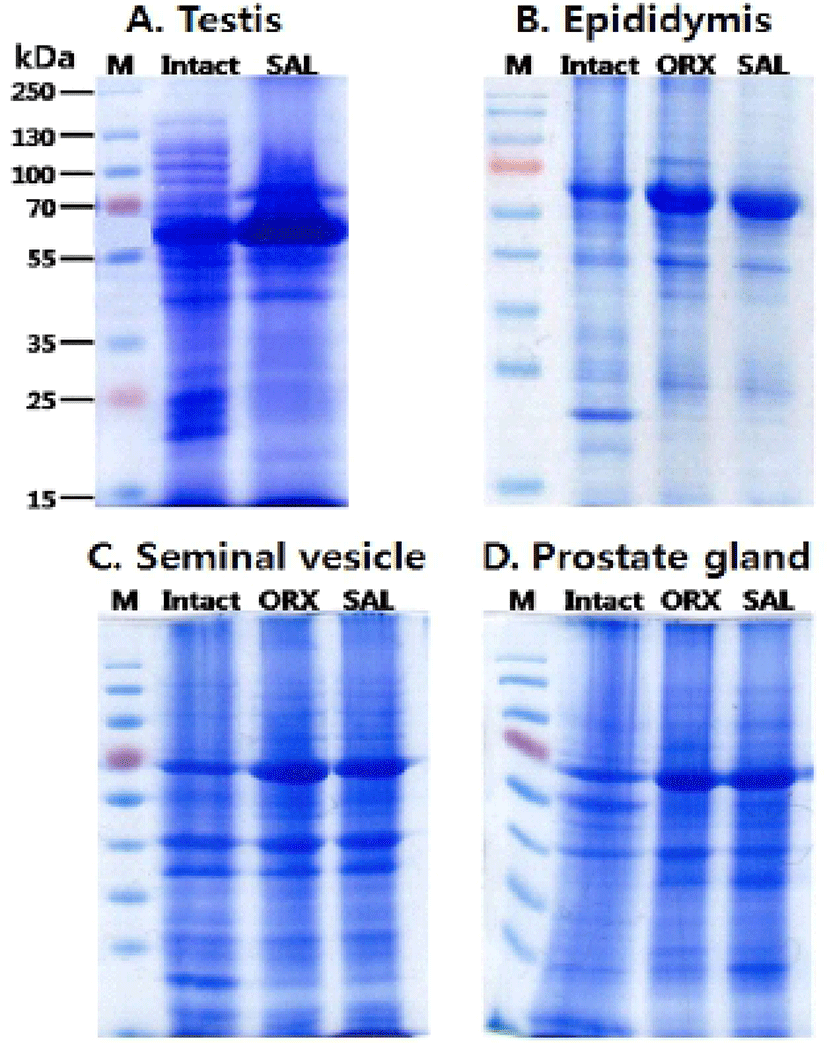
The third experiments were protein pattern analysis. The proteins from testes (A), epididymi (B), seminal vesicles (C) and prostates (D) were collected and separated using SDS PAGE gel electrophoresis (10%) (Fig. 3). When hypertonic saline was injected to the rats, the testes displayed the approximately 80 kD protein band which was not found in intact testes. Also, 90-150 kD and 20-25 kD protein bands of intact testes were disappeared and decreased in SAL group testes, respectively (Fig. 3A). When compared to the epididymal protein pattern of intact group, the patterns of ORX group and SAL group were slightly different (Fig. 3B). Approximately 72 kD bands in epididymis of both ORX group and SAL group shown higher concentration than that in intact epididymis. The concentration of 20 kD band was markedly reduced in ORX group and SAL group compared to intact group. There was no difference in the epididymal protein patterns between ORX group and SAL group. Similarly, the protein patterns of seminal vesicle and prostate from ORX group and SAL group were almost identical and shown little difference between that of intact group (Fig. 3C & D). In both ORX group and SAL group, 72 kD band of seminal vesicle had higher concentration than that in intact epididymis. Same difference of band pattern was seen in prostate proteins. The concentrations of seminal vesicle 20 kD band were markedly reduced in ORX group and SAL group compared to intact group, as shown in epididymal proteins (Fig. 3C). In the prostate samples, the concentrations of 60 kD band were markedly reduced in ORX group and SAL group compared to intact group. The concentrations of 30 kD band, on the other hand, were increased in ORX group and SAL group (Fig. 3D).
DISCUSSION
Bilateral orchidectomy, an endocrine treatment that depleting gonadal androgens, is the initially described hormonal treatment in prostate cancer and still constitutes the major castration method in developing countries (Emir et al., 2008). It is still considered as an important treatment method because of its direct and permanent effectiveness. However, infection or bleeding can become a problem and it is also time consuming, and is difficult for large-scale application, especially for controlling the population of undesirable mammals like pet animals (Jana & Samanta, 2007).
Medical or chemical castration is another well-known endocrine treatment method. An ideal chemical sterilizing agent would be one that effectively arrests spermatogenesis and androgenesis as well as libido and absence of toxic and untoward side effects (Wiebe & Barr, 1984). GnRH analogues such as degarelix are used to manage prostate cancer by desensitizing hypothalamus-pituitary-testis reproductive hormonal axis, and finally reducing the androgen action on the growth of prostate cancer cells (Steinberg, 2009). This chemical castration method is working in a reliable manner, but is very expensive.
Because simple salt solutions are inexpensive and easy to sterilization, some researchers have tested the efficacy of the solution as chemical sterilizing agent. A single bilateral intratesticular injection of CaCl2 solution resulted in induction of irreversible chemo-sterilization in male albino rats (Jana et al., 2002; Jana & Samanta, 2006). The rats exhibited the germ cell depletion as well as significant diminution in serum testosterone concentration without any pain, stress response and any toxic and unwanted side effects (Jana et al., 2006 & 2007). More recently, single dose of bilateral Intratesticular injection of hypertonic saline (20%) successfully decreased serum testosterone level which was similar to the surgical castration in the rat, showing the state of chemical castration (Emir et al., 2008; Kwak et al., 2010).
Patho-histological studies revealed that intratesticular injection of hypertonic saline resulted in severe degenerative changes in testicular seminiferous tubules and massive infiltration of immune cells. The hypertonic saline may immediately create local osmotic shock and extensive necrotic state. Necrosis is a form of cell death as a consequence of extreme physico-chemical stress which kill cells quickly and directly (Kaczmarek et al., 2013). Therefore necrosis has been considered as uncontrolled death characterized by cellular collapse, though nuclei remain intact (Krysko et al., 2008). Recent evidence indicates that some immune substances and patho-physiological conditions can induce necrosis that follows defined steps and signaling events (Vanden Berghe et al., 2010). Necroptosis, a newly coined term describing the regulated necrosis, can be mediated through a pathway that depends on the receptor-interacting protein kinase (RIPK) 1-3 (Galluzzi & Kroemer, 2011).
On the other hand, apoptosis, another type of cell death, has been widely accepted as a major mechanism of regulated cell death, employed not only upon accidental cell damage or stress but also during normal development (Nikoletopoulou et al., 2013). In the present study, hypertonic saline injection did not bring gDNA fragmentation, a key feature of apoptosis, in the accessory sex organs. The gDNA patterns in the androgen-sensitive organs were almost identical between ORX group and hypertonic saline injection group.
Using in vitro epididymis incubation and [35S] methionine incorporation technique (Jones et al., 1980), demonstrated that the radioisotope incorporation was reduced to 10% of that in normal tissues but could be restored to control levels by testosterone treatment. The authors found that the 18.5, 19.0 and 32 kD proteins in the caput part and the 47 kD protein in the cauda were preferentially regulated by androgens. In the present study, the 18.5-19 kD protein band was markedly reduced in ORX group and hypertonic saline injection group compared to intact group.
In conclusion, we demonstrated that the hypertonic saline injection could result in very similar effects on rat accessory sex organs as surgical orchidectomy.
Our study suggests that the bilateral intratesticular injection of hypertonic saline may be considered as an alternative to surgical and chemical castration methods which are widely used in the field of andrology.