INTRODUCTION
The thymus as a primary immune organ, which produces mature lymphocytes from precursor cells continuously, is of central importance in both the development and in the maintenance of the immune system (Dardenne & Savino, 1996; Fabris et al., 1997; Mocchegiani et al., 2006; Mor et al., 2001; Thyagarajan & Felten, 2002). The thymus receives stem cells from the bone marrow. The cells develop to thymocytes in the thymus and go through a series of anatomical compartments in a process termed T cell education (Anderson & Jenkinson, 2001; Petrie, 2003). Such thymopoiesis is regulated by the thymic microenvironment in which epithelial cells are the major components (Anderson et al., 2006; Chidgey et al., 2007).
Thymic epithelial cells (TECs) provide the microenvironment by direct cell contact and soluble molecules, which contribute to intrathymic thymocytes development and selection (Mor et al., 2001). TECs play key roles at multiple stages of thymocyte development where they impose self-MHC restriction on the immature thymocytes via positive selection, and aid in the elimination of autoreactive thymocytes via negative selection (Takahama, 2006). TEC defects such as genetic mutations or deletions have dramatic effects on intrathymic thymocyte development, leading to a severe immunodeficiency (Anderson et al., 2006; Chidgey et al., 2007; Holub et al., 1975). Restriction of the number of TECs has been shown a reduced number of thymocytes in the thymus (Jenkinson et al., 2007; Jenkinson et al., 2008; Revest et al., 2001). This, along with changing TEC function, is believed to be one of the major factors associated with age-dependent thymic involution (Chidgey et al., 2007).
Sex steroid hormones are known to cause thymic atrophy and loss of cellularity in both humans and animals (Clarke et al., 1994; Wang et al., 2008). Interestingly, the TECs and thymocytes express the classical ER-α and - β estrogen receptors in human and rodent (Kuiper et al., 1997; Staples et al., 1999). Estrogen exposure induces compositional changes in thymocyte populations, since estradiol treatment of mice in vivo increases the number of CD4+ SP and CD4+CD8+ DP thymocytes (Screpanti et al., 1989). In addition, estradiol causes apoptosis in thymocytes and leads to alterations in the thymocytes repertoire in such a way that the immune system may be more skewed to react strongly toward self-antigens and weakly against foreign antigens (Do et al., 2002). These facts provide a possibility that reproductive hormones such as estradiol exert pleiotropic effects on the immune system. However, little is known about the mechanism by which sex steroid hormones, such as estradiol, affect thymic atrophy and thymocyte development in the thymus. Therefore, the aim of the present study was to examine whether the change of estradiol levels in serum during the estrous cycle affects thymocyte development in the thymus.
MATERIALS AND METHODS
Six-week-old female ICR mouse were purchased from Samtako (Ohsan, Korea) and housed in groups of five per cage under controlled illumination (12:12 h light/dark cycle, lights on/off: 6 h/18 h) and temperature (22±2°C). Animals were fed a standard rodent diet and tap water ad libitum and divided into four groups that included five animals per group. Animal care and experimental procedures were approved by the Institutional Animal care and the use committee at the Seoul Women’s University in accordance with guidelines established by the Korea Food and Drug Administration.
Vaginal smears were performed on female mice to assess the progression of estrous cycle between 10:00 am and 11:00 am. Vaginal secretions were collected with a plastic pipette filled with 10 μl of normal saline (0.9% NaCl) by inserting the tip into the mouse vagina. Care was taken not to insert the pipette too deep as it may cause cervix stimulation which in turn may initiate pseudopregnancy (Hubscher et al., 2005). Saline was quickly released and immediately drawn back into it. The sample containing cells were placed on untreated glass microscopic slide and stained with wright-Giemsa stain Solution (Sigma St. Louis, MO, USA). The ingredients were observed under light microscope (YS100, Nikon, Melville, NY) with 40× objective lenses. Three types of cells were recognized: round and nucleated ones as epithelial cells; irregular ones without nucleus as cornified cells; and the little round ones as leukocytes (Byers et al., 2012; Ng et al., 2010). The proportion among them was used for the determination of the estrous cycle phases. After the stage of the estrous cycle was determined, each mouse was paired with four groups; diestrous, proestrous, estrous, and metestrous.
Blood was collected from each mouse after CO2 asphyxiation, allowed to clot at room temperature for 30 min, and then centrifuged at 6,000 rpm for 30 min to prepare serum. Serum samples were stored at –80°C until measuring estradiol concentration by ELISA assay kit (Caymen Chemical Company, MI, USA). All procedure for assay was performed following the manual in the assay kit. The sensitivity of this estradiol assay is 6.6 pg/ml.
Total RNA was isolated by using the RNA isoplus (TaKaRa Bio, Shiga, Japan) according to manufacturer’s instruction. After chloroform extraction and isopropyl alcohol precipitation, the final pellet was air dried and dissolved into RNase-free DEPC solution (TaKaRa Bio, Shiga, Japan). The RNA concentration was measured with the Nano-drop (Thermo Fisher Scientific Inc., Waltham, MA). First strand cDNA synthesis was performed in RNase-free DEPC solution containing 2 μg total RNA and 10 pmol oligo dT at 70°C for 5 min, followed by double-strand synthesis in 5X RT buffer (Invitrogen, Carlsbad, CA) with 8 mM dNTP (BIO BASIC INC., Ontario, Canada), 200 unit/μl RTase (Invitrogen, Carlsbad, CA) at 37°C for 60 min and at 72°C for 15 min.
RT-PCR was performed in buffer solution containing 3 μl of template cDNA, 5 unit/μl of Taq polymerase (BIONICS, Korea), 0.25 mM dNTPs (BIO BASIC INC., Ontario, Canada) and 10 pmol of each primer. Primer pairs were as follows: ERα forward 5'-AATTCTGAC AATCGACGCCAG-3' and reverse 5'-GTGCTTCAACA TTCTCCCTCCTC-3'; ERβ forward 5'-CTTGGTCACG TACCCCTTAC-3' and reverse 5'-GTATCGCGTCACTT TCCTTT-3'; β-actin forward 5'-CTCTTTGATGTCACG CACGATTTC-3' and reverse 5'-ATCGTGGGCCGCTCT AGGCACC-3'. The optimum temperature cycling protocol was used as 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 min, using the GenePro thermal cycler (Bioer, China). The reaction products were run on a 2% agarose gel and visualized with ethidium bromide to check the length of the amplified cDNA.
qRT-PCR was performed in a total volume of 20 μl buffer solution containing 2 μl of template cDNA, 10 μl of SYBR Green (Roche, Manheim, Germany), and 10 pmol of each primer. Primer pairs were as follows: ERα forward 5'-CATGGTCATGGTAAGTGGCA-3' and reverse 5'-TCTCTGGGCGACATTCTTCT-3'; ERβ forward 5'-TA CACTGATTCGTGGCTGGA-3' and reverse 5'-TTACG GTGTCTGGTCCTGTG-3'; 18S forward 5'-GTCTGTGA TGCCCTTAGATG-3' and reverse 5'-AGCTTATGACC CGCACTTAC-3'. The optimum temperature cycling protocol was determined to be 95°C for 5 min followed by 45 reaction cycles at 95°C for 10 sec, 60°C for 10 sec and 72°C for 10 sec using the LightCycler® 480 Real-time PCR System (Roche, Manheim, Germany).
The mice were sacrificed by CO2 asphyxiation, and then weight of the body, thymus and spleen were measured. Organ weight was calculated based on the somatic index. T cells were isolated from harvested thymi. The thymi were minced in media (RPMI+2% FBS), and then thymocytes were collected through a nylon mash (BD falcon, USA). To remove the red blood cells (RBC), RBC lysis buffer was added in the tube and left to stand for 5 min at room temperature. After washing with media (RPMI+ 2% FBS), the tube was centrifuged (1,500 rpm, 4°C, 5 min) and the supernatant discarded, and the pellet reconstituted with media (RPMI+2% FBS).
For flow cytometry analysis, 1×106 cells of thymocytes were prepared in 50 μl of staining buffer (ebioscience, USA) and incubated with blocking antibody (anti-mouse CD16/CD32, ebioscience, USA) prior to staining. Samples were labeled using combinations of the following antibodies: CD4-FITC, CD8-PE, CD44-APC, and CD25-PC7. All antibodies were purchased from ebioscience (USA). The labeled cells were analyzed by flow cytometer (CYTOMICS FC 500, Beckman coulter, USA).
RESULTS
Six week-old female ICR mice were divided into four groups according to the estrous cycle. There were no differences in the body weight among the groups. The average weight of thymi and spleens from each group was not changed during the estrous cycle. However, total number of thymocytes was significantly decreased in the proestrous phase compared to other phases (Table 1).
|
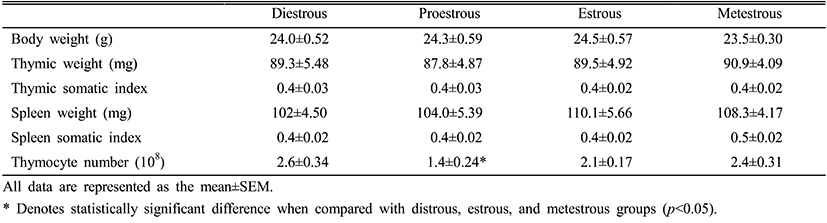
Estradiol levels in the serum during the estrous cycle were significantly increased in the proestrous and estrous phases compared to the metestrous and diestrous phases. After the proestrous phase, the estradiol levels were gradually reduced and kept at low levels in the metestrous and diestrous phases (Fig. 1).
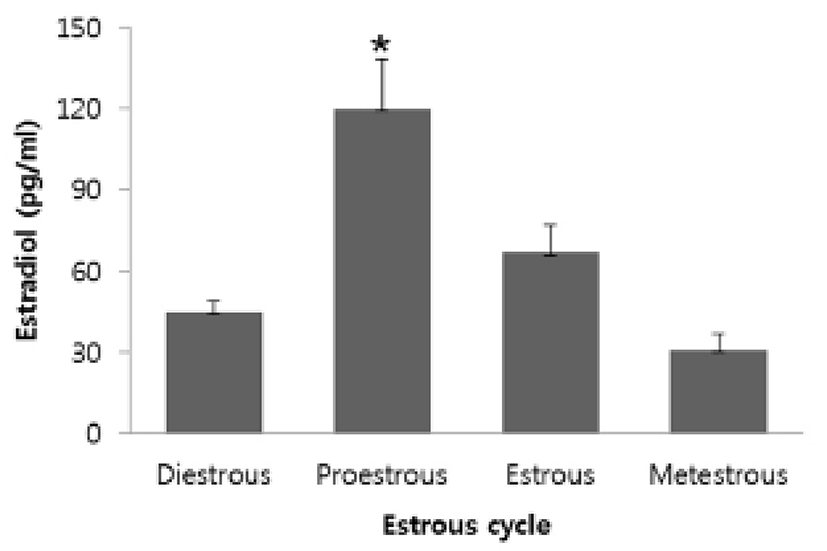
To investigate ER-α or -β mRNA expression in mouse thymus, total RNAs extracted from thymi were analyzed by RT-PCR and qRT-PCR. ER-α and -β mRNAs were expressed in thymus, thymocytes, and thymic epithelial cells. Ovary and uterus were used as positive controls (Fig. 2A). As a result of qRT-PCR, the levels of ER-β mRNA expression in the thymus were not changed during the estrous cycle, but the levels of ER-α mRNA expression were changed according to the estrous cycle showing an increase in the estrous phase (Fig. 2B).
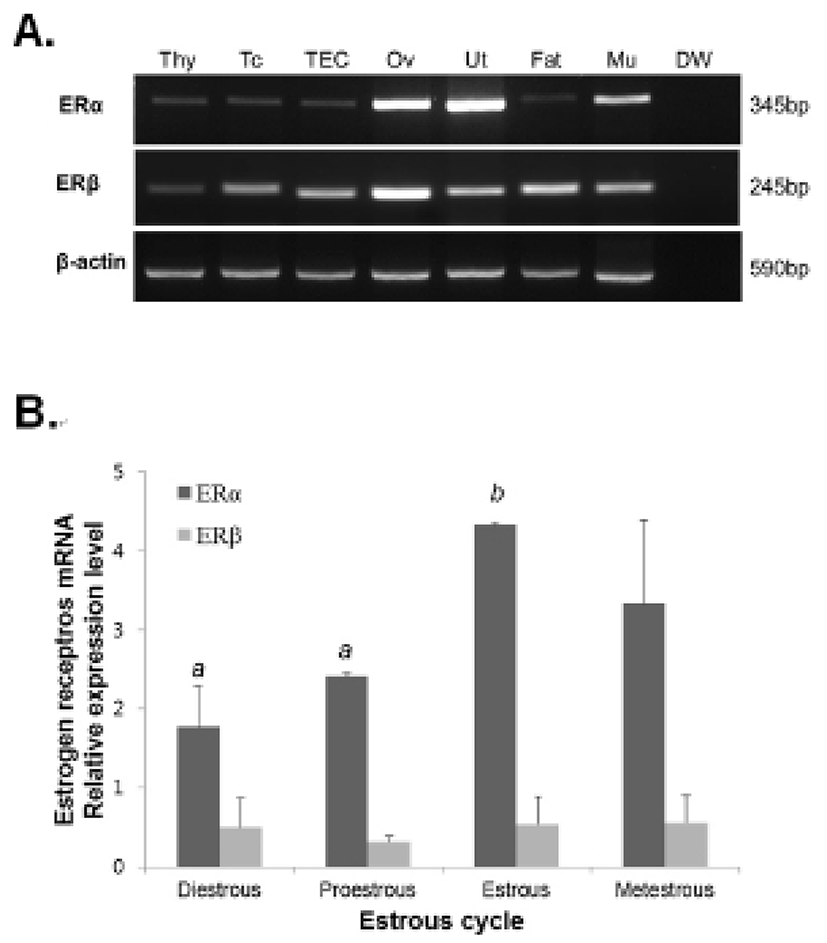
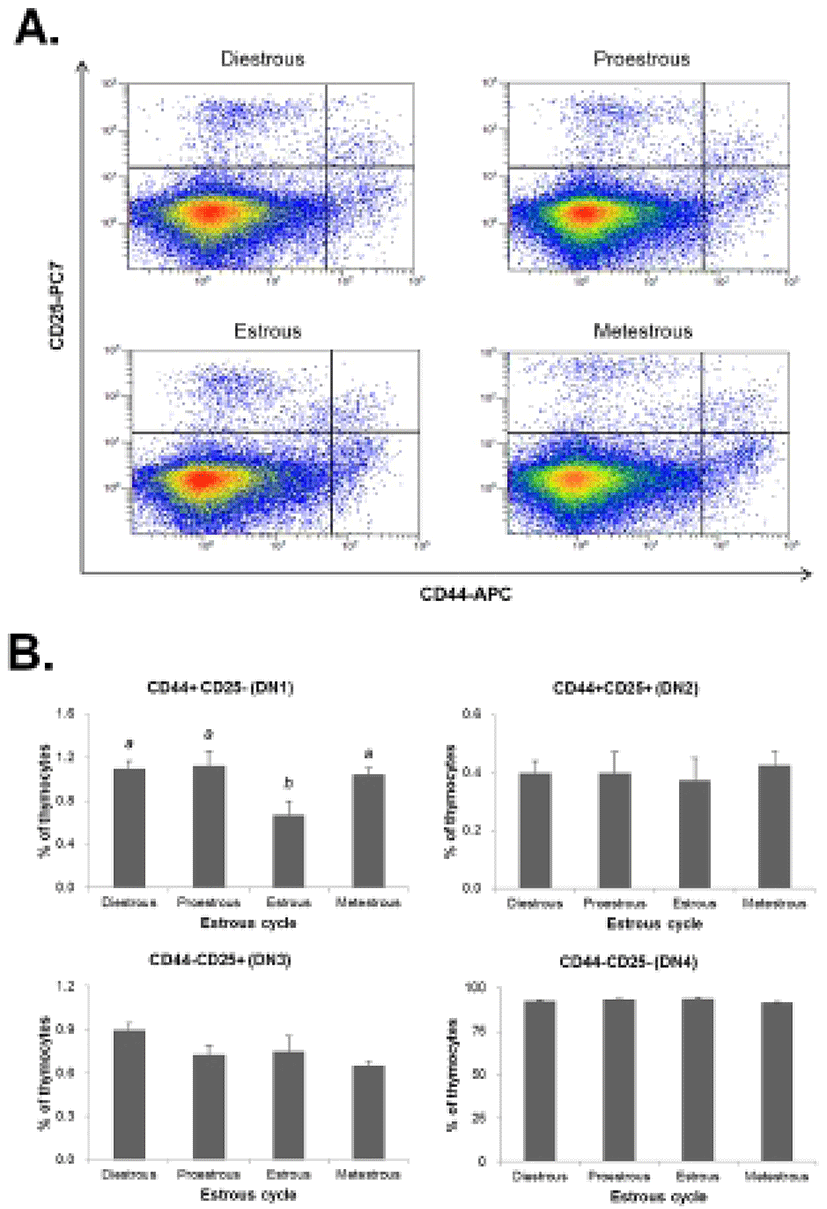
The thymocytes were labeled with CD4-FITC, CD8-PE, CD25-PC7, and CD44-APC followed by low cytometry analysis to evaluate the thymocyte development during the estrous cycle. The percentage of CD4+CD8- or CD4-CD8+ (SP) T cells was significantly increased in the proestrous phase compared to the diestrous phase, and then remained at high levels in the estrous and metestrous phases. The percentage of CD4-CD8- (DN) T cells was also significantly raised in the proestrous phase compared to the diestrous phase, and then remained at high levels in the estrous and metestrous phases. In contrast, the percentage of CD4+CD8+ (DP) T cells was significantly decreased in the proestrous phase compared to the diestrous phase, and then remained at low levels in the estrous and metestrous phases (Fig. 3). We also analyzed the distribution of DN T cells which are in early stage of thymocyte development. The percentage of CD44+CD25- (DN1) T cells was significantly decreased in the estrous phase compared to other phases, whereas CD44+CD25+ (DN2), CD44-CD25+ (DN3), and CD44-CD25- (DN4) percentages were not changed during the estrous cycle (Fig. 4).
DISCUSSION
Previous reports have shown that treatment with estradiol triggers thymic atrophy and apoptosis of thymocytes (Do et al., 2002; Holladay et al., 1993; Okasha et al., 2001; Silverstone et al., 1994; Staples et al., 1999; Yao & Hou, 2004). Many studies have demonstrated that estradiol alters intrathymic thymocyte development (Brunelli et al., 1992; Rijhsinghani et al., 1996; Screpanti et al., 1989). Moreover, it has been reported that estradiol might affect lymphocytes indirectly by mediating estrogenic effects on thymic epithelial cell (TEC), which have higher expression of estradiol receptor (Luster et al., 1984). These data provide the possibility that estradiol may be involved in the production of cytokines and growth factors secreted by the thymic epithelial cells. Although many hypotheses about the effects of estradiol on thymus have been proposed, the precise mechanism remains unclear so far. Therefore, the present study aimed to examine whether the change of estradiol and progesterone levels in the serum during the estrous cycle affects thymocyte development in the thymus.
We demonstrated here that the number of thymocytes is decreased in the proestrous phase, whereas the serum estradiol levels are significantly increased in the proestrous phase. This result suggests that the increased estradiol level may cause apoptosis of thymocytes, resulting in reduction of the number of thymocytes in the thymus. It is known that estradiol induces apoptosis in thymocytes and leads to alterations in the thymocytes repertoire in such a way that the immune system may be more skewed to react strongly toward self-antigens and weakly against foreign antigens (Do et al., 2002). ER-deficient mice have smaller thymus than the wild-type littermates and estradiol induced thymus atrophy was reduced in ERdeficient mice (Erlandsson et al., 2001; Staples et al., 1999). Estradiol exerts its effects via genomic signaling which is mediated by interaction with nuclear estrogen receptors. The pleiotropic effects of estrogen are mediated by two classic estrogen receptors known as estrogen receptor ER-α and -β. It has been reported that ER-α is expressed in both thymocytes and TECs (Kawashima et al., 1992). We also demonstrated here that ER-α and -β are expressed in both thymocytes and TECs. The development and maturation of thymocytes in the thymus is influenced by TECs which have ER-α and -β (Boyd et al., 1993; Ritter & Boyd, 1993). The TECs is also known to produce cytokines essential for expansion and development of thymocytes (Di Santo & Rodewald, 1998; Malek et al., 1999). These results propose the possibility that increased estradiol levels in the proestrous phase suppress the secretion of cytokines in TECs causing the inhibition of thymocyte development.
Next, we analyzed the population of thymocytes in the thymus during the estrous cycle to determine the effect of estradiol on thymocyte development. The percentage of CD4+CD8+ double-positive (DP) T cells was significantly decreased in the proestrous phase, but CD4+CD8- or CD4-CD8+ (SP) T cells were significantly increased. In addition, the percentage of CD44+CD25- (DN1) T cells was significantly decreased in the estrous phase, whereas the percentages of CD44+CD25+ (DN2), CD44-CD25+ (DN3), and CD44-CD25- (DN4) were not changed during the estrous cycle. It is suggested that high levels of estradiol may block the development of thymocyte at the transition stage from CD4-CD8- (DN) to CD4+CD8+ (DP) stage leading to dysfunction of thymus and reduction of immune response. It has been reported that thymocyte development responds directly to sex steroid hormones, especially estrogen which regulates the immune cell differentiation and functions (Couse & Korach, 1999; Yellayi et al., 2002). It was also known to induce atrophy and phenotypic alterations when exposed to elevated levels of estrogen (Staples et al., 1999).
Taken together, our study suggested that a change in estradiol levels during the estrous cycle may be involved in the regulation of thymocyte differentiation in the mouse thymus. Although it is not clear yet whether the inhibition of thymocyte differentiation is due to estradiol binding to thymocytes or TECs, estradiol seams to play a critical role in thymocyte differentiation. We need more study on the function of other sex steroid hormones with estradiol on thymocyte differentiation and the mechanism by which estradiol inhibits thymocyte differentiation.