INTRODUCTION
Sex of bivalves is classified into gonochorism and hermaphroditism, and hermaphroditism is further divided into synchronous (simultaneous or functional) and asynchronous (sequential) hermaphroditism. The asynchronous hermaphroditism signifies sex reversal in accordance with seasons, resultantly (Heller, 1993; Gosling, 2004).
Indirect evidence of sex reversal in bivalves is a change in the sex ratio of a population with shell size (Orton, 1933; Galtsoff, 1964; Guo et al., 1998; Eversole, 2001; Gosling, 2004; Lee et al., 2012). Sex reversal of bivalves has been reported in the Ostreidae (Orton, 1933; Galtsoff, 1964; Thompson et al., 1996; Guo et al., 1998; Gosling, 2004), Pectinidae (Osanai, 1975; Ventilla, 1982) and Veneridae (Eversole, 2001; Lee et al., 2013).
This study attempted to present indirect evidence on the sex reversal of Sinonovacula constricta and Gomphina veneriformis by examining the changes in sex ratio with shell length and also to suggest basic information to definitively investigate their sex.
MATERIALS AND METHODS
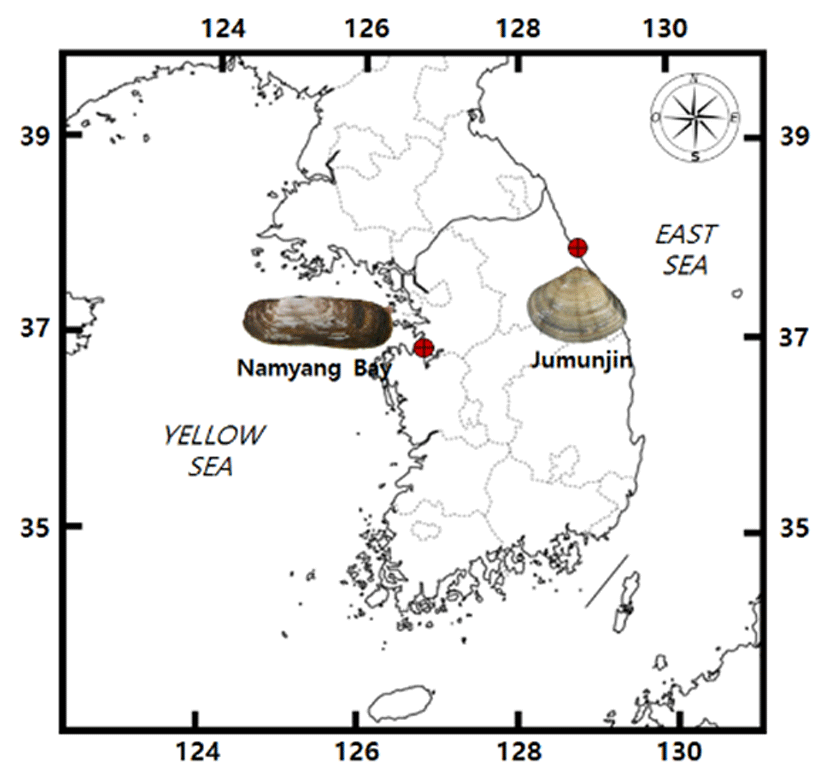
Sinonovacula constricta used for sex ratio analysis was collected from the Namyang Bay on the western coast of Korea from April 1997 to March 1998. Total number of S. constricta used for sex ratio analysis was 100 animals (shell length 26.5-95.0 mm) (Table 1). Gomphina veneriformis used for sex ratio analysis was collected from Joomoonjin on the eastern coast of Korea from October 2005 to August 2007. Total number of the clam used for sex ratio analysis was 2385 (shell length 15.1-70.0 mm) (Table 2).
The sex ratios were computed with the following equation to illustrate the percentage (%) of the females.
Female (%) = [Female/Female+Male]×100
Histological techniques were used to confirm the sex and gonadal development of each specimen. Specimen preparation for light microscopy was performed according to the methodology of Drury & Wallington (1980). The clams were dissected, and their visceral mass, which included the gonad, was fixed in aqueous Bouin’s solution for 18 h and rinsed in running water for 24 h and then dehydrated through a graded ethanol series (70-100%). The tissues were embedded in paraplast (McCormick, USA). Embedded tissues were sectioned at 4-6 um thickness using a microtome (RM2235, Leica, Germany). Samples were stained with Mayer’s hematoxylin-0.5% eosin (H-E).
RESULTS
The female proportion (F/F+M) for the entire population of S. constricta was 41.0%. However, sex ratio was found to differ when the clams were divided into groups according to shell length in 10.0 mm intervals. Sex ratio displayed the results of the tendency of increase in the proportion of female as increased in shell length (Table 1).
The female proportion (F/F+M) for the entire population of G. veneriformis was 50.0%. However, sex ratio was found to differ when the clams were divided into groups according to shell length in 5.0 mm intervals. Although there was tendency of increase in the proportion of female as shell length increased (Table 2).
DISCUSSION
There was a case in which sexual dimorphism in bivalves was reported in Dysnomiacap saeformis and D. brevidens (Mackie, 1984). However, since it is difficult to distinguish sex macroscopically, histological analysis of gonads remains the only conclusive method. However, this method cannot be carried out without killing the organism (Gosling, 2004).
Although the sex of the majority of bivalves cannot be identified externally, the ratio of females to males is the same and they are generally gonochoristic (Gosling, 2004). Hermaphroditism (synchronous or asynchronous) in bivalves was recorded in 13 families among 117 analyzed families (Heller, 1993).
Synchronous hermaphroditism is the simultaneous release of eggs and sperm by one organism during the same season. Asynchronous hermaphrodities function first as one sex, then as another. Asynchronous hermaphrodities can be protandrous, protogynous or can alternate sexuality (Heller, 1993). Synchronous hermaphroditism in bivalves has been reported in Anodonta grandis (Van Der Schalie & Locke, 1941) and Elliptio (Heard, 1979) of Unionidae; Argopecten irradians (Sastry, 1979), Chlamyso percularis (Sastry, 1979) and Pecten latiauritus (Mackie, 1984) of Pectinidae; Fulviamutica of Cardiidae (Chang & Lee, 1982); Corbicula fluminea of Corbiculidae (Britton & Morton, 1982); and Gemma spp. of Veneridae (Mackie, 1984). Asynchronous hermaphroditism in bivalves has been reported in Crassostrear ivularis, C. madrasensis, Saccostrea glomerata and S. cucculata (Asif, 1979), Ostreaedulis (Sastry, 1979; Gosling, 2004), C. virginica (Mackie, 1984; Gosling, 2004) of Ostreidae and Mercenaria mercenaria (Sastry, 1979; Gosling, 2004) and Ruditapes philippinarum (Lee et al., 2013) of Veneridae.
Although sex reversal has been reported for other bivalves, such as Patinopecten yessoensis (Osanai, 1975; Ventilla, 1982), sex reversal studies have traditionally been conducted on oysters. Historically, the literature points to the higher proportion of males at the early stage, with increasing proportion of females arising through sex reversal at older stages of oysters (Orton, 1933; Coe, 1934; Galtsoff, 1964; Thompson et al., 1996; Guo et al., 1998).
European oyster, O. edulis exhibits between 10-16% male to female sex reversal in the first year, and approximately 50% male to female sex reversal in the second year, respectively (Orton, 1933). They mature as males initially and undergo sex reversal into females after the first discharge of sperm, while sex reversal is repeated throughout their life cycle (Walne, 1974). Quahog clam, M. mercenaria, become sexually mature at less than one year old, developing first as males but changing to an equal sex ratio in the second year (Menzel, 1989) (Fig. 1, 2).
Sex of S. comstricta has been reported to be gonochoristic (Han et al., 2005) also reported the sex of G. veneriformis to be gonochoristic (Park et al., 2003). These conclusions on the sex of S. comstricta (Han et al., 2005) and G. veneriformis (Park et al., 2003) may have been reached because of the limited investigation undertaken by these authors some stage of the life cycle of S. constricta and G. veneriformis rather than continuous tracking of the reproductive life-history over a long period.
However, in this study, sex reversal is forecasted for S. constricta and G. veneriformis based on the results of analysis of changes in the sex ratio with shell sizes. Therefore, the possibility that the sex of these species is asynchronous hermaphroditism is very high.
Another indication of indirect evidence is the fact that large number of bivalves have an inactive stage during the reproductive cycle and classification of morphological sex is impossible as the gonad tissue is completely degenerated during this period (inactive stage) (García-Domínguez et al., 1994; Behzadi et al., 1997; Villalejo-Fuerte & García-Domínguez, 1998; Park et al., 2003). In the case of these bivalves, there is possibility that they may develop as different sex when the next reproductive cycle begins.
C. virginica and R. philippinarum are protandric species with an increase in the proportion of females through sex reversal as it ages. Sex reversal normally occurs when the gonad is undifferentiated between spawning seasons (Thompson et al., 1996; Lee et al., 2013). Although the sex of the scallop Patinopecten yessoensis, is male during the young stage of less than one year, sex is thereafter reversed to female. Histological analysis showed that their sex is not reversed during the inactive stage of their reproductive cycle, but is rather reversed through continuous stages during the reproductive cycle (Osanai, 1975).
From the results of Park et al. (2003), which histologically analyzed the gonad through all the stages of the reproductive cycle of G. veneriformis, no observation could be made of the histological characteristics of the gonad that illustrated the process of sex reversal at any stage of the reproductive cycle. Therefore, it is determined that the sex reversal of G. veneriformis occurs during the inactive stage following the spawning season.
Two factors, namely genetic and environmental, are involved in the changes in sex ratio and sex determination of bivalves. In the case of Pomacea canaliculata, C. gigas and Mytilus sp., an oligogenic sex determination method was used, which is a mechanism that results in highly diversified sex ratio and sex determination by small number of genes (Guo et al., 1998; Yusa, 2007). Factors such as temperature, food availability and day length are involved in environmental sex determination of bivalves (Yusa, 2007). Changes in sex ratio due to exposure to pollutants, such as EDCs and heavy metals during the inactive season, have also been reported in Mya arenaria (Gagné et al., 2005), G. veneriformis (Lee & Park, 2007; Ju et al., 2009) and Scapharca broughtonii (Lee et al., 2009).
As the result of this study, although the sex reversal of S. constricta and G. veneriformisis predicted, additional researches are necessary for presentation of direct evidences for and environmental factors of sex reversal in these species.
