INTRODUCTION
Far Eastern catfish, Silurus asotus is classified Siluriformes and Siluridae (Park et al., 2004). The fish is warm water fish so it inhabits 20~27°C and it lives soft ground. And the fish is nocturnal so it active night and it is carnivorous fish so it mostly predates not only crustaceans but also aquatic animals. Far Eastern catfish grow about 20~50 cm per a year. The fish live all of Korea rivers, and international habitat is Japan, China and Taiwan. They are delicious and highly nutritious so utilized by edible fish (Park et al., 2004).
To develop aquaculture technique of Far Eastern catfish in Korea, biological techniques were studied such as temperature dependent mitotic cycle, triploid, gynogenetic diploid, breeding and rearing, spermatozoa structure, inducing ovulation, and karyokinesis in embryos (Choi et al., 1992; Kwon et al., 1996; Kwon et al., 1998; Kim et al., 2001; Im et al., 2000, 2001, 2002; Park & Im, 2001). Besides, occurrences of abnormal fishes were studied in previous researches (Nguyen et al., 2008; Lijalad & Powell, 2009; Roo et al., 2009; Boglino et al., 2011; Haga et al., 2011). Decreasing abnormal fishes is very important for optimum production. Therefore, deformity of Far Eastern catfish must be studied for development of aquaculture technique.
Deformities are observed all of organisms, and it justly appears to fish. Fish have various deformities, representatively skeletal deformities. Skeletal deformities were observed such as gilthead sea bream, Sparus aurata, Senegalese sole, Solea senegalensis, and Atlantic salmon, Salmo salar (Gavaia et al., 2009; Georgakopoulou et al., 2010; Boglino et al., 2011). Until now, the deformity of fish were studied about body shape, jaw deformity, vertebral deformity and skeletal deformity (Nguyen et al., 2008; Lijalad & Powell, 2009; Boglino et al., 2011; Haga et al., 2011).
Skeletal deformities have many risks for fish, such as swimming performance, feed efficiency, limit of the feed, infectious disease and lethality (Andrades et al., 1996; Imsland et al., 2006; Le Vay et al., 2007; Lijalad & Powell, 2009; Puvanendran et al., 2009). Furthermore, deformities make concern from fish welfare (Ashley, 2007).
Deformities were found in indoor rearing conditions in 2 years, this study began to determine what type of deformity is found. So, this experiment measured dimension of morphometric characteristic (Park et al., 2004), length, weight, gonadosomatic index (GSI), hepatosomatic index (HSI) and viscera index (VI). In addition, this study observed Far Eastern catfish’s skeletal structure using X-ray system.
MATERIALS AND METHODS
In July 2012, using this experimental fish, 60 Far Eastern catfish, Silurus asotus were produced of seeds and sampling at the Fishery Genetics and Breeding Sciences Laboratory, Korea Maritime and Ocean University, Busan, Korea. Deformity was chosen to have cosmetic differences.
A Far Eastern catfish’s tank that was a circulation filter water tank was under fluorescent light (1,000 lux) conditions. The light was on from 6:00 to 18:00. So, environmental condition was kept temperature, pH, DO (dissolved oxygen), photoperiod, ammonia, nitric acid, nitrous acid and conductivity (Table 1). Water was changed fresh water once a week.
Test parameters were analyzed at 1 month interval during 2 years. The lux of fluorescence lights that used in this experiment were 1,000 lux. Temperature, pH, dissolved oxygen and salinity were measured using an oxygen measurement electrode and a multi-data logger system (Oxyguard, Denmark). Ammonia, nitric acid, nitrous acid and conductivity were measured using spectrophotometer (DR2800, HACH, Loveland, Colorado, USA). The values are means of triplicate groups (n=20).
Assorted feed was made by Chenhajeil Feed© (Daejeon, Korea). Feed composed following: Table 2. It was given ad libidum twice a day (9:00 and 18:00), by hand.
| Nutrition | Content (%) |
|---|---|
| Crude protein | 40.0 |
| Crude fat | 5.0 |
| Crude fiber | 5.0 |
| Ash | 14.0 |
| Calcium | 1.0 |
| Phosphorus | 1.0 |
| Mineral premix*2 | 1.0 |
| Vitamin premix*3 | 1.0 |
Vitamin premix contained the following amount which were diluted in cellulose (g kg 1 mix): L-ascorbic acid, 121.2; DL-α-tocopheryl acetate, 18.8; thiamin hydrochloride, 2.7; riboflavin, 9.1; pyridoxine hydrochloride, 1.8; niacin, 36.4; Ca-D-pantothenate, 12.7; myo-inositol, 181.8; Dbiotin, 0.27; folic acid, 0.68; p-aminobenzoic acid, 18.2; menadione, 1.8; retinyl acetate, 0.73; cholecalciferol, 0.003; cyanocobalamin, 0.003.
Mineral premix contained the following ingredients (g kg 1 premix): NaCl, 43.3; MgSO4·7H2O, 136.5; NaH2PO4 ·2H2O, 86.9; KH2PO4, 239.0; CaH4(PO4)·2H2O, 135.3; ferric citrate, 29.6; ZnSO4·7H2O, 21.9; Ca-lactate, 304.0; CuCl, 0.2; AlCl3·6H2O, 0.15; KI, 0.15; Na2Se2O3, 0.01; MnSO4 ·H2O, 2.0; CoCl2·6H2O, 1.0.
In August 2014, Far Eastern catfish were stopped feeding on the day before study. After Far Eastern catfish samples were killed by overdosing with clove oil (Sigma, USA). They were taken pictured at above, side and head under copystand (Polaroid Dongwon Co., Korea). After taken picture, measured standard length (Ls) were measured as cm unit with ruler and body weight, gonad weight, liver weight and intestine weight were measured as g unit with electric balance (g; SHIMADZU AW320, Japan). Gonadosomatic index (GSI), hepatosomatic weight (HSI), and viscera index (VI) were calculated as GSI = (gonad weight/body weight) ×100, HSI = (liver weight/body weight) ×100, and VI = (intestine weight/body weight) ×100.
After analysis, Far Eastern catfish were fixed 1 day by 10% formalin, the day after changed new 10% formalin and measured their morphometric characteristics. Measuring morphometric characteristics were paralleled with truss dimension and classical dimension, and they classified with Ls, DALAD, DPDPL, HPLAA, HALAV, HALOP, DAAPO, DAUPO, DALAD, CH, BDAA, BDMA, HWOP, HL, HARL, DAHR(L)B, DAHR(L)U and DAHR(L)L (Table 3 and Fig. 1; Park et al., 2001, 2004).
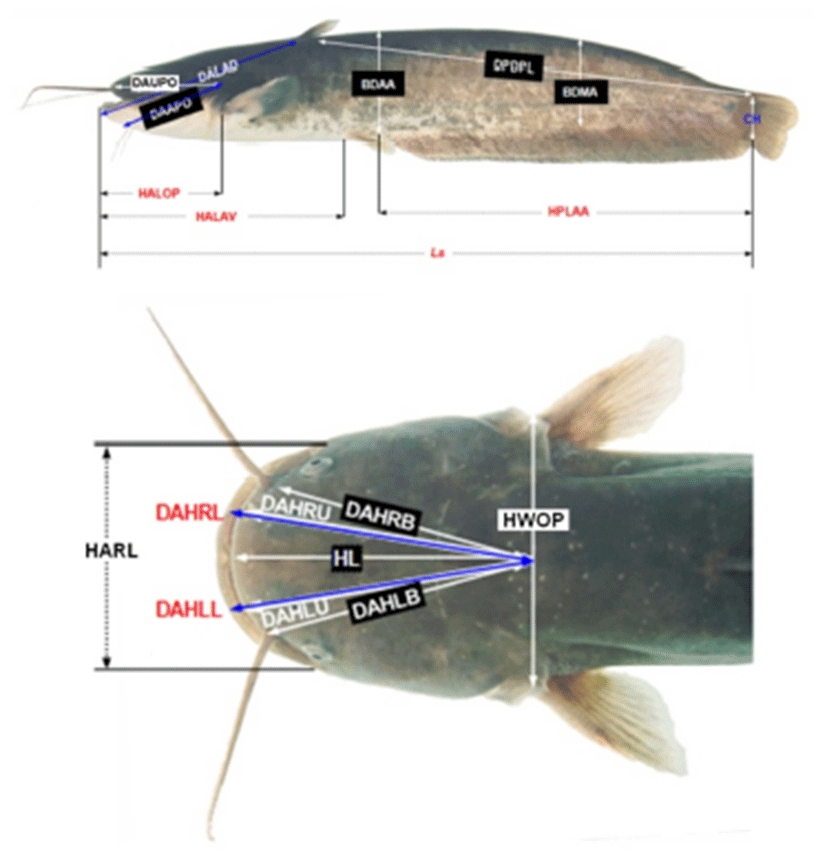
Each dimension is demonstrated in Fig. 1 (Modified from Park et al., 2004).
Measured morphometric characteristics were switch Arc sin square root. DALAD, DPDPL, HPLAA, HALAV, HALOP, DAAPO, DAUPO, DALAD, CH, BDAA and HWOP were analyzed relative value (%) about Ls, BDMA was Ls and BDAA, HARL was HWOP, DAHRB was DAHLB, DAHRU was DAHLU and DAHRL was DAHLL (Park et al., 2004).
X-rays (CR Director Maker, KODAK, USA) took to see detail skeletal deformities including normal individual for formalin fixed Far Eastern catfish. The picture was taken Taejong Animal Hospital (Busan, Korea) and X-ray photographing mode is computer radiography (CR). Pictures were taken at above, lateral side and head region.
RESULTS
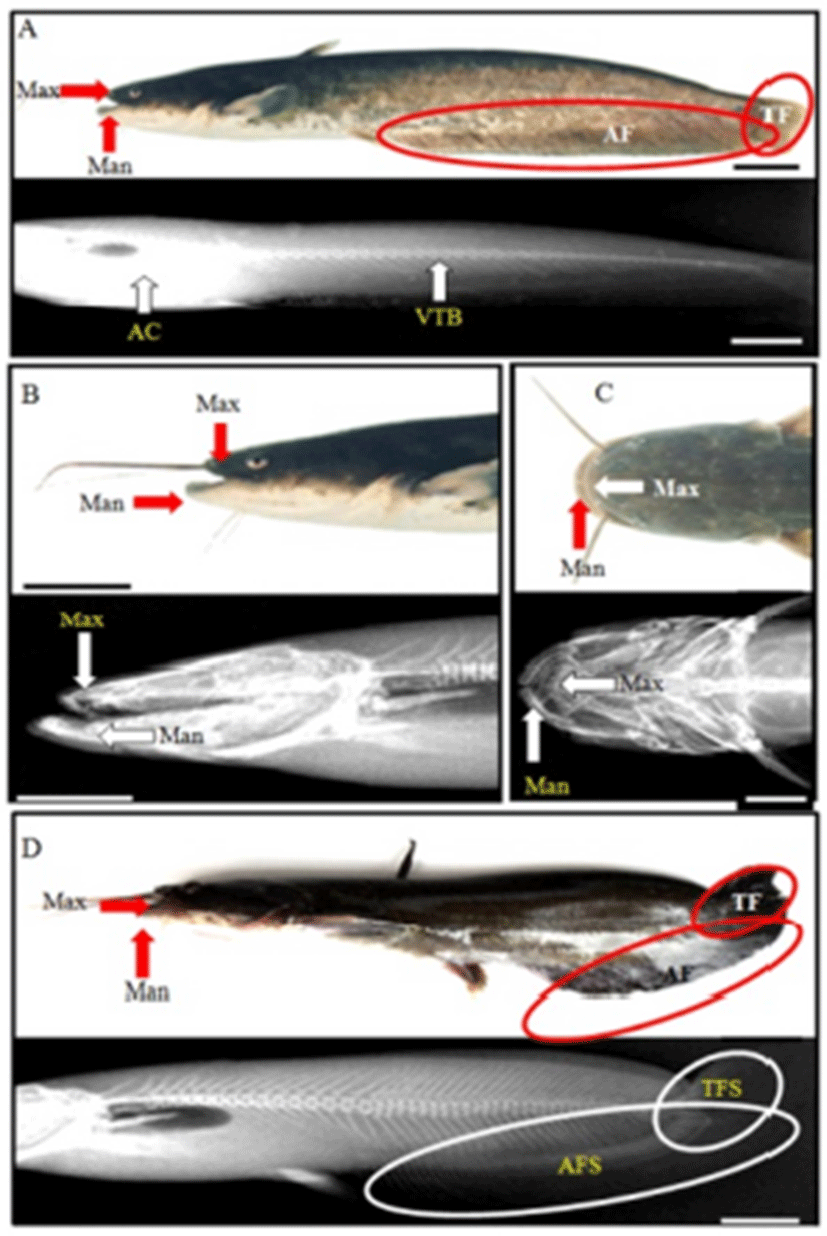
In this experiment, we observed 4 types (spinal curvature, jawbone’s luxation, abnormality of upper lip, and malocclusion; Tables 4 and 5, Figs. 2 and 3) of deformity in Far Eastern catfish, Silurus asotus. The samples of standard length in other deformities, except for spinal deformity, were similar to normal sample, and the sample of spinal deformity had large odds between them (Table 4). Spinal deformity was so small that the weight was light in comparison with others. The abnormal rates of each group were 1/60 (1.6%) and GSI, and HSI and VI made no odds. Therefore, other deformities, except for spinal deformity, did not influence on growth and production of eggs.
| Total samples (n=60) | Normal samples (n=56) | Types of normal and skeletal deformity*2 | ||||
|---|---|---|---|---|---|---|
| S(n=1) | J(n=1) | U(n=1) | M(n=1) | |||
| Standard length(mm) | 461±8.2 | 461±8.2 | 182.2 | 454.5 | 463.9 | 441.3 |
| Body weight(g) | 341±9.8 | 341±9.8 | 122.4 | 354.1 | 367.2 | 349.1 |
| Abnormal rate(%) | 4/60(6.5) | 0/56(0.0) | 1/60(1.6) | 1/60(1.6) | 1/60(1.6) | 1/60(1.6) |
| GSI*3 | 6.5±1.27 | 6.5±1.00 | 6.4 | 6.9 | 6.1 | 6.6 |
| HSI*4 | 3.2±1.54 | 3.3±0.86 | 3.0 | 3.4 | 3.9 | 3.1 |
| VI*5 | 5.1±1.41 | 5.0±0.79 | 4.8 | 4.8 | 4.9 | 4.1 |
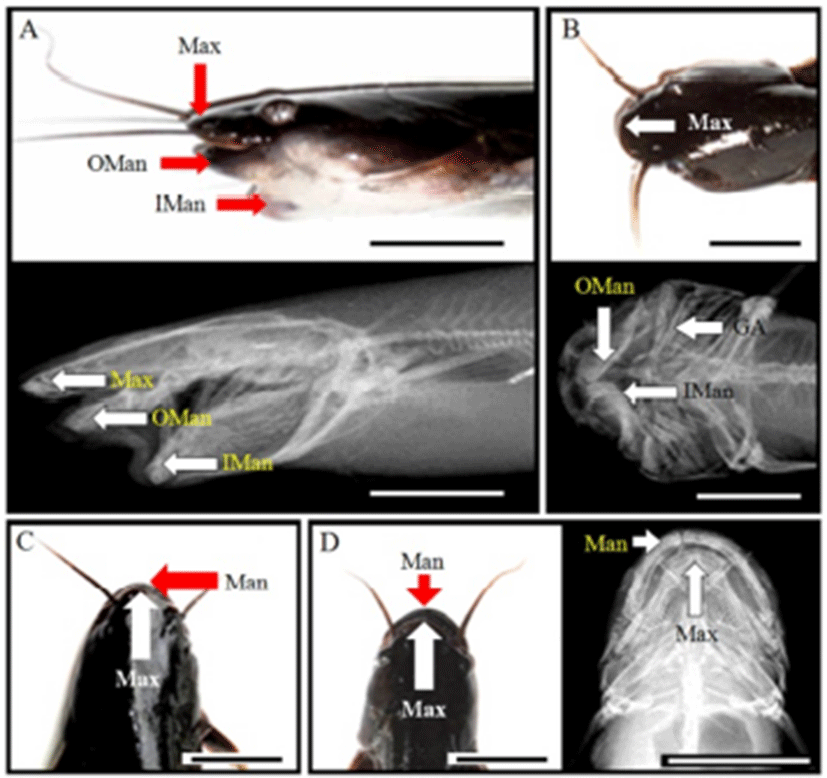
Normal samples had long and straight anal fin; however, spinal curvature had short anal fin (Fig. 2). Also, normal sample’s tail fin was dropped, but spinal curvature was up and was seen to be connected with anal fin. Furthermore, tail was stubby. This skeletal structure indicated that the body was relatively round than normal sample, so it had fin spine upper. From spinal deformity, significant differences were found; DPDPL/Ls (62.8), HPLAA/Ls (44.1), and CH/Ls (2.4) were smaller than others, and BDMA/BDAA (83.6) was larger (P>0.05; Table 5). This means that DPDPL, HPLAA, and CH were relatively long about Ls, and BDMA was larger than others (P>0.05). In X-ray (Fig. 3), normal sample has straight vertebra, short maxilla, and long mandible. In contrast, deformities are different. Spinal deformity does not have straight vertebra and the tail fin spine is pointing up.
| Morphometric dimension (%) | Normal samples (n=56) | Types of skeletal deformity*2 | |||
|---|---|---|---|---|---|
| S (n=1) | J (n=1) | U (n=1) | M (n=1) | ||
| DALAD/Ls | 32.3±2.78 | 31.5 | 32.1 | 32.4 | 31.8 |
| DPDPL/Ls | 71.7±3.74 | 62.8* | 72.1 | 72.8 | 71.2 |
| HPLAA/Ls | 56.9±2.18 | 44.1* | 55.4 | 56.4 | 56.1 |
| HALAV/Ls | 37.5±1.16 | 37.4 | 37.9 | 38.0 | 37.6 |
| HALOP/Ls | 21.6±0.25 | 21.0 | 22.4 | 21.9 | 22.1 |
| DAAPO/Ls | 14.6±1.05 | 14.8 | 16.8* | 14.9 | 14.1 |
| DAUPO/Ls | 19.5±0.49 | 19.4 | 19.9 | 19.4 | 20.0 |
| DALAD/Ls | 16.7±0.40 | 16.5 | 14.1* | 16.8 | 16.0 |
| CH/Ls | 5.8±0.59 | 2.4* | 5.9 | 6.1 | 5.6 |
| BDAA/Ls | 16.3±0.63 | 17.1 | 16.4 | 16.8 | 16.1 |
| BDMA/Ls | 12.5±0.99 | 14.3* | 12.8 | 12.5 | 12.5 |
| BDMA/BDAA | 76.5±3.16 | 83.6* | 78.0 | 74.4 | 77.6 |
| HWOP/Ls | 15.9±0.67 | 15.4 | 15.9 | 16.4 | 15.6 |
| HARL/HWOP | 81.1±4.28 | 83.1 | 70.8* | 78.5 | 81.4 |
| DAHRB/DAHLB | 100.1±3.07 | 101.1 | 98.9 | 91.1* | 100.2 |
| DAHRU/DAHLU | 100.4±2.91 | 100.5 | 99.7 | 90.8* | 100.8 |
| DAHRL/DAHLL | 100.2±4.88 | 100.7 | 100.9 | 100.1 | 101.9* |
As shown in Fig. 2, normal sample has a short maxilla and a long mandible. However, jaw deformity has a long maxilla and short mandibles. It has a small and asymmetric head, and the eyes are protruded. So, only the maxilla can be observed when viewing from above. Furthermore, jaw deformity has 2 mandibles when saw side: outer side of mandible and inner side of mandible. Inner side of mandible is seen as a lump. As shown in Table 5, differences in the jaw deformity are DAAPO/Ls (16.8), DALAD/Ls (14.1), and HARL/HWOP (70.8). DAAPO and DALAD were so short that the data were too small (P>0.05). Furthermore, HARL was too small to show low data (P>0.05). In this deformity, standard length was too short, so DAAPO/Ls and DALAD/Ls (14.1) were calculated to be low. Furthermore, this saw head had one flexion, so HARL/HWOP was calculated to be low. For the deformity in the saw side, there were three jawbones, and the inner mandible was connected to grill bones. Head bone was short and unstable, and saw gill arch (Fig. 3).
One has lip that is higher, and the right lip is shorter than the left lip (Fig. 2). The right lip was back over, so it saw inner. From the upper lip deformity, DAHLB & DAHRB and DAHLU & DAHRU were not in balance, so it made a difference (P>0.05; Table 5). DAHLB/DAHRB and DAHLU/DAHRU at about 100 were normal, but it was too small (DAHLB/DAHRB: 91.1, DAHLU/DAHRU: 90.8). This catfish’s left maxilla was longer than the right mandible, so it made odds.
As shown in Fig. 2, Malocclusion was seen inside of the mouth, and the head was asymmetric. Malocclusion’s DHARL/DHALL was bigger than other, it means that there is no balance at jaw (P>0.05; Table 5). Malocclusion does not match the balance of the left maxilla and right maxilla, and it was observed that the right maxilla is longer than the left maxilla. External shape could not be seen, but the skeletal structure saw what side of maxilla was leaned. Consequently, I saw right maxilla was leaned.
DISCUSSION
In fish, skeletal deformity was indentified with infection, physical damage, deficiency of nutrition, and development of bladder (Officer et al., 1995). It is similar to these 3 types of skeletal deformities, which re lordosis, kyphosis, and scoliosis (Hibiya, 1982).
In this study, most fatal deformity in Far Eastern catfish, Silurus asotus is accompanied by spinal curvature. There was low datas, such as the weight was low at approximately 119.6 g and standard length was low at about 159.8 mm. It means that the spinal deformity delayed growth of the Far Eastern catfish. Jaw deformities didn’t have odds in standard length and weight, and it means that these deformities are not fatal when eaten. GSI, his, and VI had no odds, and it means that the deformities didn’t have risk regarding the gonad, liver, and intestine. Furthermore, in spinal curvature, these weights were as small as the catfish’s weight.
In this study, we saw 4 deformities, which is part of all deformities. A study indicates that the deformities are mostly about appearance and skeletal deformity (Yoo et al., 2003; Fjelldal et al., 2007; Nguyen et al., 2008; Boglino et al., 2011; Haga et al., 2011). Haga et al. (2011) found the loss of caudal fin and supernumerary caudal fin rays that were observed in Japanese flounder, Paralichthys olivaceus. Changes in the morphology of fins seem to be related to stages; earlier treatment induces fin loss but it later induces supernumerary fin rays (Haga et al., 2011).
So, it is necessary to consider why deformities occur and how many types of deformities exist. The deformities are caused by environmental conditions (temperature, salinity, pH, photoperiod, dissolved oxygen and light intensity: Lee & Menu, 1981; Steingraeber & Gingerich, 1991; Georgakopoulou et al., 2010). Besides, it has other causes such as diets, ions, or radioactivity (Fjelldal et al., 2007; Nguyen et al., 2008; Roo et al., 2009; Boglino et al., 2011). When vitamin A is insufficient, there is fatal risk such as in the development of skeletons (Haga et al., 2011). Furthermore, essential fatty acids are necessary to elucidate bone deformities of fish, and HUFA level leads to different effect of deformities (Roo et al., 2009). Recently, significant research activity has been focused on the nutrigenomics and proteomics approaches in fish physiology and molecular biology (Panserat & Kaushik, 2010).
Consequently, by giving the same environmental condition and diet, deformities were identified. So, it is necessary to study why the Far Eastern catfish deformities occurs the reason for these deformities, which are likely to be caused by the increases in any condition, and ways to prevent the appearances of these deformities. Furthermore, we need to elucidate the importance of environment, diet, ions, and radioactivity. If we stand the cause of deformities, it can reduce the incidence of malformations to produce more superior Far Eastern catfish.
