INTRODUCTION
Red spotted grouper, E. akaara is one of the most popular and important grouper species for aquaculture in south-east and east Asian countries including Japan, Taiwan, Hong Kong and Indonesia thanks to its fast growth and high market value. Despite the significant economic value, large scale seed production techniques for this species have not been completely developed to date. One of the major difficulties in seed production is associated with the reproductive characteristics of grouper species.
Groupers that belong to the genus of Epinephelus are known to be protogynous hermaphrodites that first differentiates into female and changes to male later (Tan & Tan, 1974; Brusle- Sicard et al., 1992; Shapiro et al., 1993; Bhandari et al., 2003; Alam & Nakamura, 2007; Murata et al., 2009; Liu & Sadovy, 2009; Sao et al., 2012). Natural sex change of E. akaara from female to male occurs 5 to 6 years old (Li et al., 2006; Li et al., 2007). Brood stocks of E. akaara, however, often show male preponderance within a few years under artificial conditions, suggesting bi-directional sex change (Tanaka et al., 1990; Okumura, 2010). These are indicative of poor understanding on the process of sex differentiation and sex reversal in this species.
In many fish, hormonal treatment can induce functional sex change from female to male as well as produce allmale or all-female population of young fish (Yamazaki, 1983; Hunter & Donaldson, 1983). Red spotted grouper is one of those species that requires the help of hormonal treatment to achieve stable supply of male and female gametes for successful seed production. Tanaka et al. (1990) have found male fish in 1 year old group of this species, implying the possibility of primary male differentiation. However, hormonal induction of primary male differentiation in this species has not been satisfactory so far (Lee et al., 2014). Understanding on the process of early gonadal differentiation would improve sex reversal technique for this species. Therefore, we investigated early development of gonad for more than 3 months from hatching based on histological changes, and analyzed the relationship between fish size and gonadal differentiation in red spotted grouper, E. akaara.
MATERIAL & METHODS
Red spotted grouper, E. akaara were collected twice from Cheongsol aquaculture farm in Muan-gun, Jeonnam, Korea, and transported to a laboratory in Sunmoon University (38 and 40 DPH, day post hatching). Fish were reared at 27 ± 1°C and 31 ± 1‰ in indoor tanks. Photoperiod was maintained at 14 hours light : 10 hours dark (14L:10D). Fish were fed a commercial diet (40 to 80 DPH: Ottohime, Japan; 80 to 130 DPH: Myungsun, Korea) ad libitum twice a day. Sampling was carried out 5 days between 40 to 105 DPH. Additionally, fish at 38, 120, 130 DPH were sampled further.
These fish were anesthetized by 50 ppm benzocaine (Sigma) and measured for body weight (g) and total length (mm). Whole body samples were first fixed in 10% formalin for about 12 h. After elimination of head and tail from the fixed fish, the remaining tissues were further fixed in 10% formalin again for about 12 h. The fixed tissues were then dehydrated with ascending alcoholic series, cleared in xylene, and embedded in paraffin wax. Tissues were cut to a thickness of 5~8 μm, stained with haematoxylin & eosin and observed under light microscope (DM500, Leica, Germany).
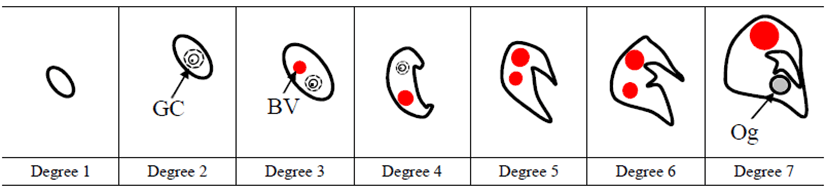
Gonadal differentiation was categorized as undifferentiated gonad phase, differentiated gonad phase and ovarian phase on the basis of two protuberant aggregations of somatic cells in gonadal primordium (onset of ovarian cavity formation) and appearance of ovarian cavity and oogonia (onset of ovarian phase). To analyze correlation between gonadal differentiation degree and fish size, gonadal differentiation degree was further divided into seven steps and granted score one to seven then we carried out linear regression analysis.
RESULTS
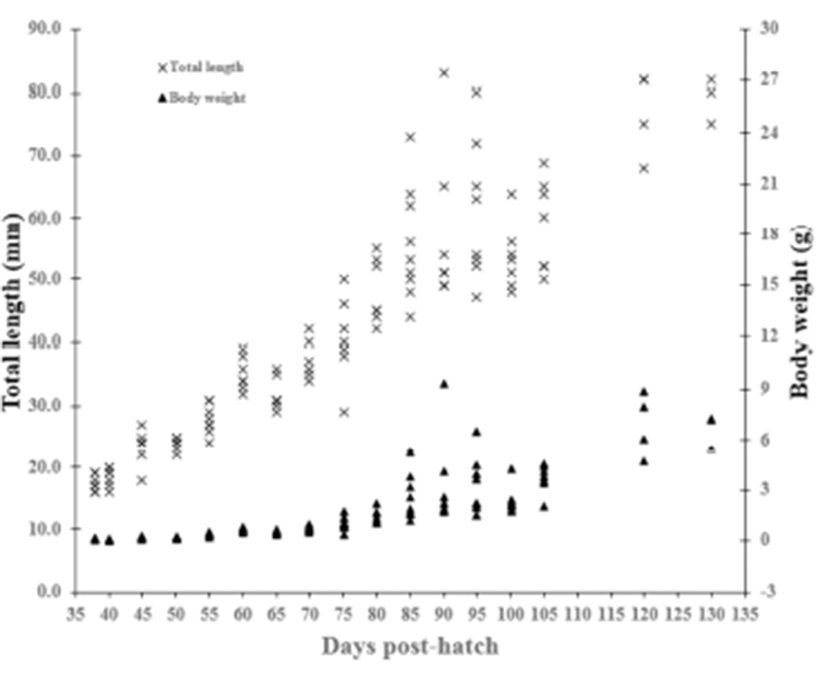
In undifferentiated gonadal phase of red spotted grouper, E. akaara (from 38 to 60 DPH), total length and body weight ranged from 17.4 to 35.1 mm and 0.1 to 0.6 g, respectively. In initially differentiated gonad (65 DPH), total length and body weight were 31.3 ± 0.78 mm and 0.5 ± 0.04 g, respectively. In differentiated gonadal phase (65 to 130 DPH), total length and body weight ranged from 31.3 to 79.0 mm and 0.5 to 6.9 g, respectively (Fig. 1).
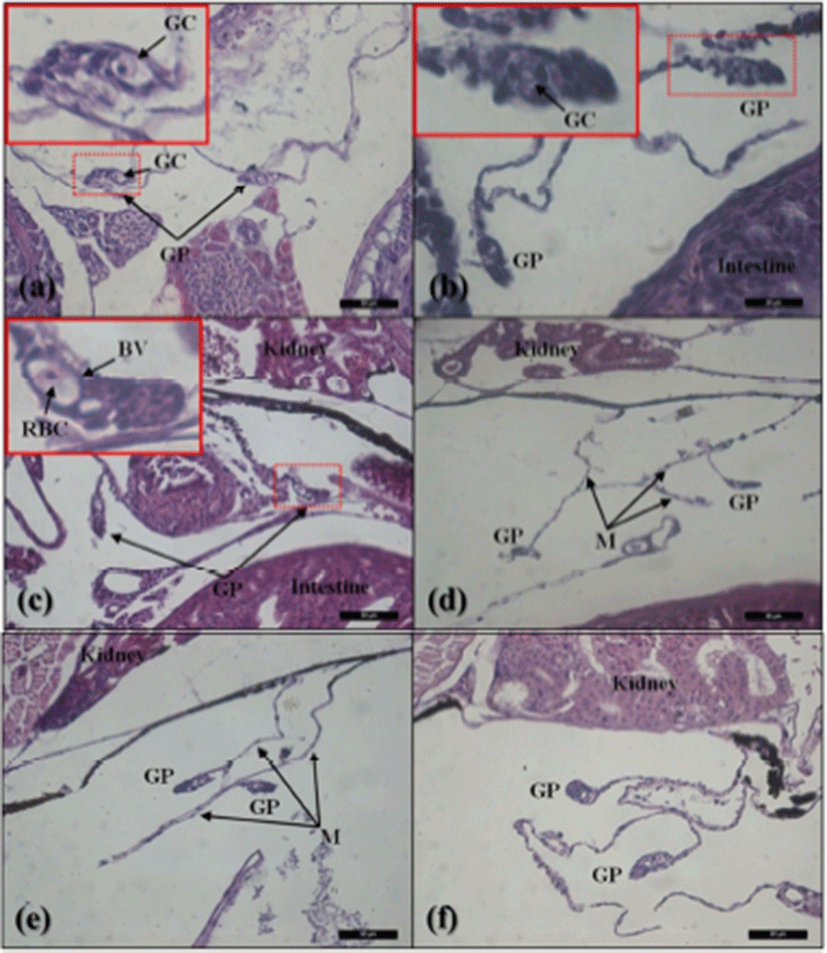
The gonadal primordium of red spotted grouper, E. akaara was observed at 38 DPH (Fig. 2). A pair of gonadal primordium was located in the middle part of mesentery, immediately below the kidney in the body cavity. Gonadal primordium of 38, 40 DPH consisted of germ cells surrounded by a few somatic cells. The blood vessel was observed in the gonadal primordium at 45 DPH. The number of somatic cells and size of gonadal primordium increased age-dependently up to 60 DPH. In the gonadal primordium of all fish at 65 DPH, formation of ovarian cavity was obvious by two protuberant aggregations of somatic cells (Fig. 3). In most of the samples up to 130 DPH, the two elongating walls of somatic cells developed further but not connected to each other to complete the ovarian cavity. However, completed ovarian cavity and oogonia were first observed in the gonad of one fish sample at 105 DPH.
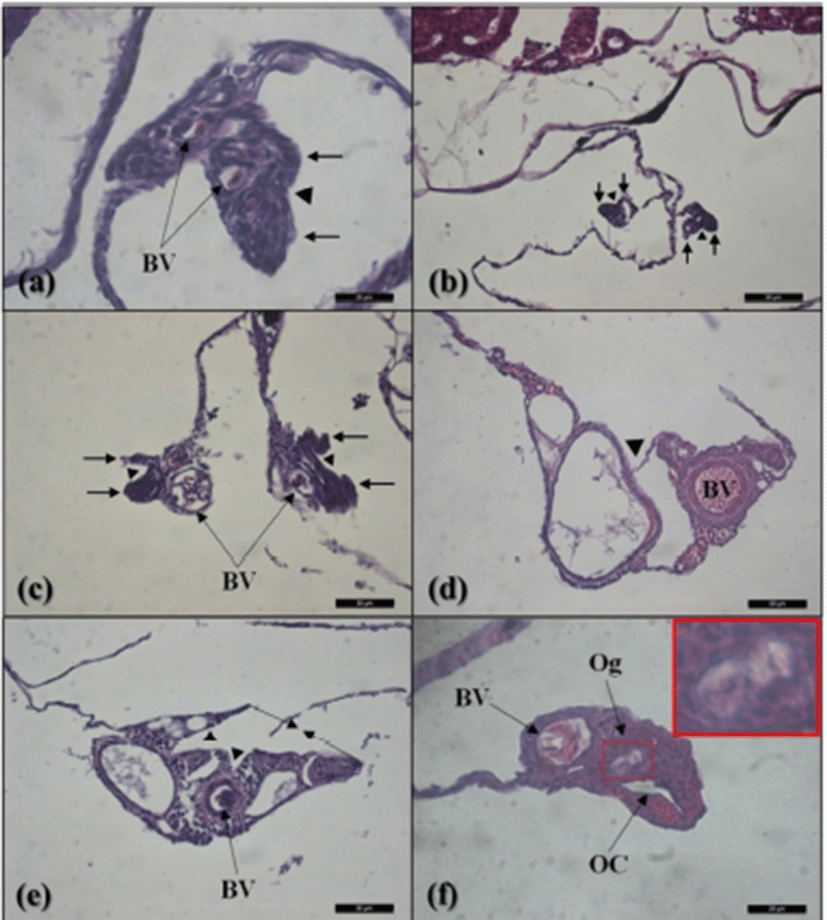
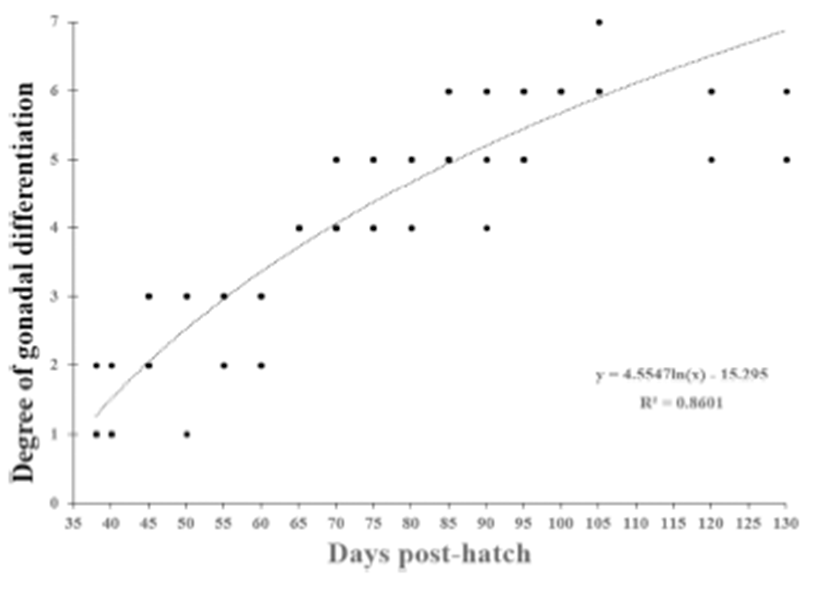
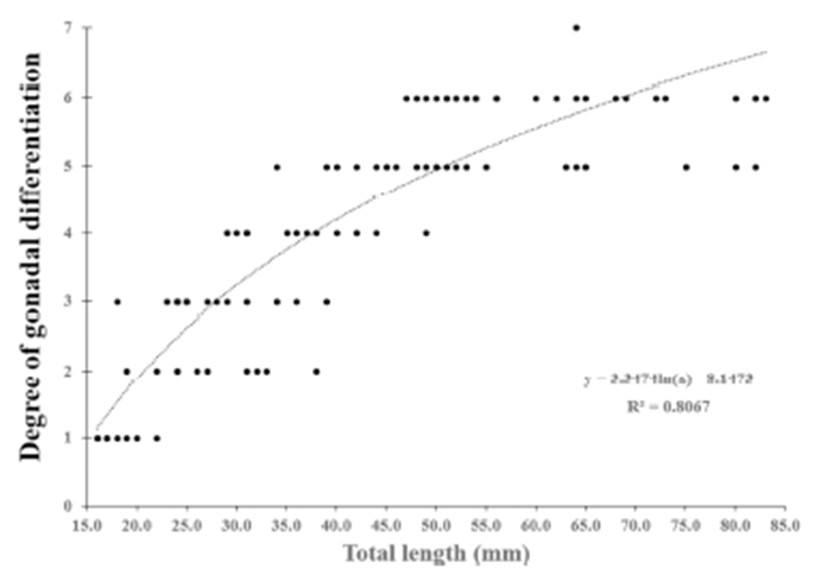
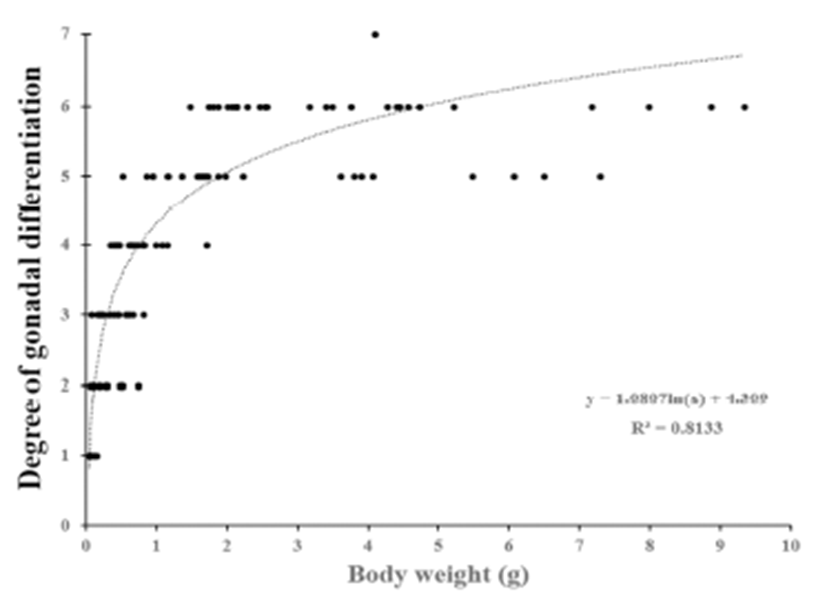
We divided the degree of gonadal differentiation into seven (Fig. 4). The correlation of gonadal differentiation degree with DPH was positive and statistically significant (R2 = 0.8607, P < 0.05) (Fig. 5). However, correlation between fish size (total length and body weight) and the degree of gonadal differentiation were not statistically associated (P > 0.05) (Fig. 6 and 7).
DISCUSSION
A pair of gonadal primordium has already existed underneath the kidney in the posterior part of the body cavity at 38 DPH when this study began. In other grouper species, appearance of gonadal primordium was first observed at 20 DPH in longtooth grouper, E. bruneus; at 18 DPH in malabar grouper, E. malabaricus; at 3 weeks after hatching (WAH) in orange spotted grouper, E. coioides (Murata et al., 2009; Liu & Sadovy, 2009; Sao et al., 2012). This result indicates that the first appearance of gonadal primordium in red spotted grouper could be much earlier than 38 DPH.
Germ cells as well as gonadal primordium were found at 38 DPH in this species. The timing of germ cell appearance in grouper depends on fish species (Murata et al., 2009; Liu & Sadovy, 2009; Sao et al., 2012). The appearance of germ cell was found at 40 to 50 DPH in longtooth grouper, E. bruneus; at 39 DPH in malabar grouper, E. malabaricus; at 7 WAH in orange spotted grouper, E. coioides. Appearance of germ cell in red spotted grouper seems to be earlier than other grouper species mentioned here.
Morphological characteristic of ovarian cavity formation was first observed in gonadal primordium of all fish at 65 DPH in this species. This morphological characteristic is similar to what occurs in the onset of ovarian cavity formation in other teleost fish such as Cottus bairdii, Oreochromis aureus, O. mossambicus, Carassius auratus, O. niloticus (Hann, 1927; Eckstein & Spira, 1965; Nakamura, 1978; Nakamura & Nagahama, 1985). The morphological characteristic was also very similar to other grouper species such as malabar grouper, E. malabaricus; longtooth grouper, E. bruneus; orange spotted grouper, E. coioides (Murata et al., 2009; Liu & Sadovy, 2009; Sao et al., 2012).
In the present study, completed ovarian cavity and oogonia were first observed in the gonad of one fish sample at 105 DPH. Nakamura et al. (1998) reported that physiological sex determination in germ cells occurs in synchrony with gonadal sex differentiation. Appearance of ovarian cavity and oogonia means completion of ovarian sex differentiation. However, ovarian cavity was not completed in many fish until 130 DPH. In other grouper, the ovarian cavity formation was reported to be completed at 110 to 130 DPH in longtooth grouper, E. bruneus and at 144 DPH in malabar grouper, E. malabaricus (Murata et al., 2009; Sao et al., 2012). Liu & Sadovy, (2009) have also shown that the completed ovarian cavity was first observed in orange spotted grouper, E. coioides and humpback grouper, Cromoleptes altivelis at 16 to 30 WAH and at 22 WAH respectively.
The correlation of gonadal differentiation degree with DPH was statistically significant while correlations of gonadal differentiation degree with fish size (total length and body weight) were not. It could be expected that the bigger fish may have more differentiated gonad than the smaller fish at the same age. However, analysis from the present study suggests that the progress of gonadal differentiation depends on the age of fish (DPH) rather than the size of fish in this species at least until the completion of ovarian cavity.
The gonads of all fish examined in this studied differentiated into ovarian structure. This confirms red spotted grouper is protogynous species, supporting previous studies (Sadovy de Mitcheson & Liu, 2008). Serranidae hermaphrodites are classified as Serranus type, Rypticus - Anthias type and Epinephelus type depending on gonadal development and sex change type (Smith, 1965). Sexual patterns of red spotted grouper, E. akaara belong to Epinephelus genus which is protogynous hermaphrodites with bidirectional sex change (Sadovy de Mitcheson & Liu, 2008). Protogynous hermaphroditism is divided into monandry and diandry (Reinboth, 1967). However, protogynous hermaphroditism in red spotted grouper is yet to be further clarified.
In fish, the induction of artificial sex change is most effective during gonadal sex differentiation (Yamamoto, 1969; Nakamura & Takahashi, 1973; Nakamura et al., 1998). Lee et al. (2014) have shown that induction of primary male differentiation can be possible by a treatment with 17α-methyltestosterone in red spotted grouper at 70 DPH. In the present study, undifferentiated gonadal phase was up to 60 DPH, and ovarian differentiation began between at 60 to 65 DPH. Based on this result, it can be suggested that induction of primary male differentiation could be more successfully applied at around 60 DPH in this species.
