INTRODUCTION
The digestive tract is basically a tube that courses through the body. However, the structure and functional characteristics of digestive system vary widely among species (Suyehiro, 1941) and are established by genetic determinants such that they are matched to wide diversity of feeding habits and environmental conditions (Buddington & Krogdahl, 2004). Digestive system functions are regulated by several digestive hormones and substances. In digestive actions, cholecystokinin (CCK) and mucus-secreting goblet cell are play important physiologic action in the intestine of vertebrates including fish. CCK is one of the gastrointestinal hormones and the most abundant neurotransmitter peptides in the brain and the intestine. CCK plays a crucial role in the regulation of pancreatic enzyme secretion (Jensen & Holmgren, 1985; Einarsson et al., 1997; Johnsen, 1998), gallbladder contraction (Aldman et al., 1992; Einarsson et al., 1997; Liddle, 1997), regulation of amino acid and sugar transport (Verspohl & Ammon, 1987) and regulates intestine peristalsis (Olsson et al., 1999).
The mucus-secreting goblet cells in the fish digestive tract produce a lubricant for the mucosal surface, which prevents the mucous membrane from being damaged by physical or chemical action and protects it from the actions of digestive enzymes (Allen et al., 1986). The mucus secretion of goblet cells in vertebrates including fish plays important roles in absorption of easily digestible substances (Osman & Caceci, 1991; Domeneghini et al., 2005).
In this study, we investigated the distributions and characteristics of CCK-producing cells and mucus-secreting goblet cells with respect to stomach fish and stomachless fish of individual species in order to provide a basis for understanding the digestive physiology and biology of the Gobiidae. Specifically, the subjects of this study were the hairychin goby, Sagamia geneionema fish which is stomachless fish, the gluttonous goby, Chasmichthys gulosus, the trident goby, Tridentiger obscurus and the giurine goby, Rhinogobius giurinus which are stomach fish (Hur et al., 2015). S. geneionema and C. gulosus inhabit in seawater, and T. obscurus and R. giurinus inhabit brackish waters in Jeju coastal area.
MATERIALS AND METHODS
S. geneionema (n=20, 6.7±0.2 cm in total length) and C. gulosus (n=20, 12.3±0.2 cm in total length) were collected from the Hamdeok-ri coastal area of Jeju Island, South Korea. T. obscurus (n=20, 8.9±0.4 cm in total length) and R. giurinus (n=20, 8.4±0.2 cm in total length) were collected from an estuary at Cheonjiyeon waterfall on Jeju Island.
The collected specimens were anaesthetized with 2-phenoxyethanol, and the entire digestive tract, from the esophagus to the anus, was removed from the body cavity.
Samples from six parts of the digestive tract (esophagus, stomach, anterior intestine portion, mid intestine portion, posterior intestine portion, rectum) were fixed in Bouin's solution, dehydrated in a graded series of ethanol, embedded in paraffin, and then cut in 5 μm cross and longitudinal sections (Fig. 1).
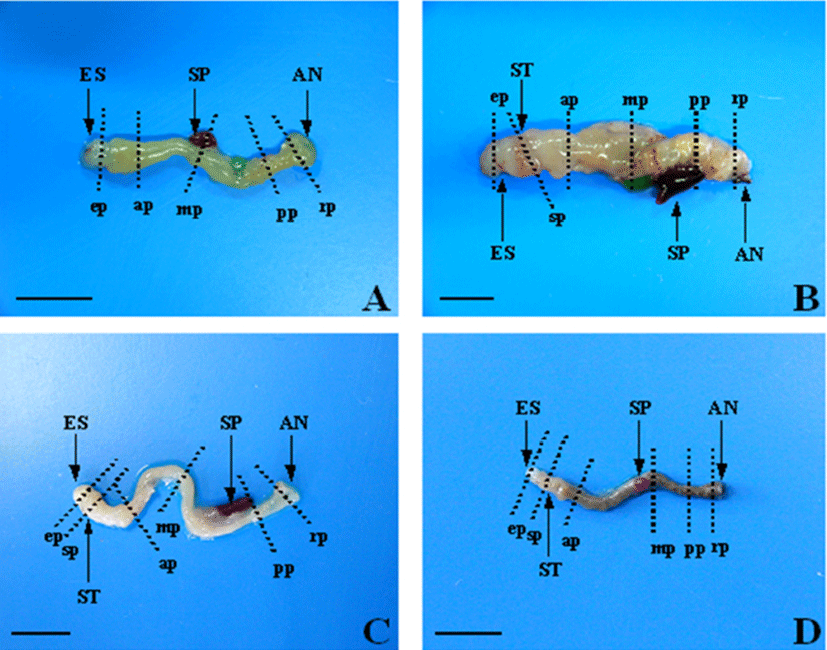
Sections were stained with Hansen's hematoxylin and 0.5% eosin (HE) for general histological observations, and were stained with Alcian blue (AB) at pH 2.5 and periodicacid- Schiff (PAS) for observations of mucus-secreting goblet cells.
Characteristics of the goblet cells from different regions of the digestive tract was carried out using a light microscope (Carl Zeiss, HBO 50) with Image scope 2.3 (Image Line, Inc.) software.
Six fish from each species were processed for immunohistochemistry. CCK-Producing cells were visualized using the avidin-biotin complex (ABC) method (Hsu et al., 1981). Microscope slides were coated with poly-L-lysine to promote tissue section adherence.
After the sections were deparaffinized and rehydrated, they were incubated in 0.5 mM periodic acid to block endogenous peroxides. After three rinses in 0.1 M phosphatebuffered saline (PBS; pH 7.2), nonspecific binding was blocked with 10% normal goat serum in PBS for 15 min. The solution was blotted off from the slides, primary CCK antiserum (1:8000, Sigma, Israel) was added, and the slides were incubated for 22 h at 4°C in a moist chamber. After three rinses in PBS, the sections were incubated for 50 min at room temperature (around 23°C) in anti-rabbit goat serum (IgG, Vector, USA) diluted 1:200 in PBS. After three more rinses in PBS, the sections were incubated for 1 h at room temperature (around 23°C) with streptavidin labeled peroxidase diluted 1:100. After three rinses in PBS, the DAB substrate system (Vector, USA) was added for peroxidase reactions. All samples were prepared on a clean bench and incubated in a moist chamber. After immunostaining, the sections were mounted in Canada balsam solution (Sigma, USA), and CCK-IR cells were observed by light microscopy (Carl Zeiss, HBO 50) with Image scope 2.3 (Image Line, Inc.) software.
RESULTS
According to the location of the digestive tract and situation in that region, different regional distributions and relative frequencies of CCK-producing cells were observed. These differences are shown in Table 1.
| Numbers of CCK-producing cells/ tissue section | ||||
|---|---|---|---|---|
| S. geneionema | C. gulosus | T. obscrus | R. giurinus | |
| Esophagus | N.D. | N.D. | N.D. | N.D. |
| Stomach | · | N.D. | N.D. | N.D. |
| Anterior intestine | 124±27a | 175±5b | 26±15 | 83±23a |
| Mid intestine | 30±8b | 38±26a | 23±10 | 10±4b |
| Posterior intestine | 8±1b | N.D. | N.D. | N.D. |
| Rectum | 4±1b | N.D. | N.D. | N.D. |
CCK-producing cells were not detected in the esophagus, but were found with various frequencies in the anterior intestine portion and extending to the rectum. The numbers of CCK-producing cells were recorded from anterior intestine portion (124±27), mid intestine portion (30±8), posterior intestine portion (8±1) and rectum (4±1) (Table 1). Thus, the highest frequency was observed in the anterior intestine portion, with decreasing frequencies toward the rectum (P<0.05).
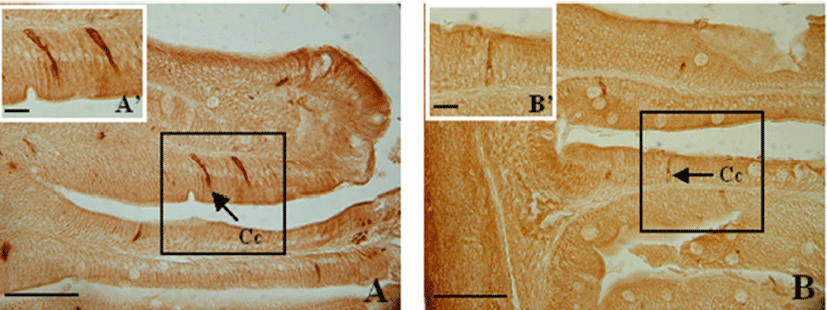
Spindle-shaped and a few spherical cells were dispersed among the epithelial cells. CCK- producing cells extended from the epithelium to the intestinal lumen, with their thin apexes pointing toward the lumen (Fig. 2).
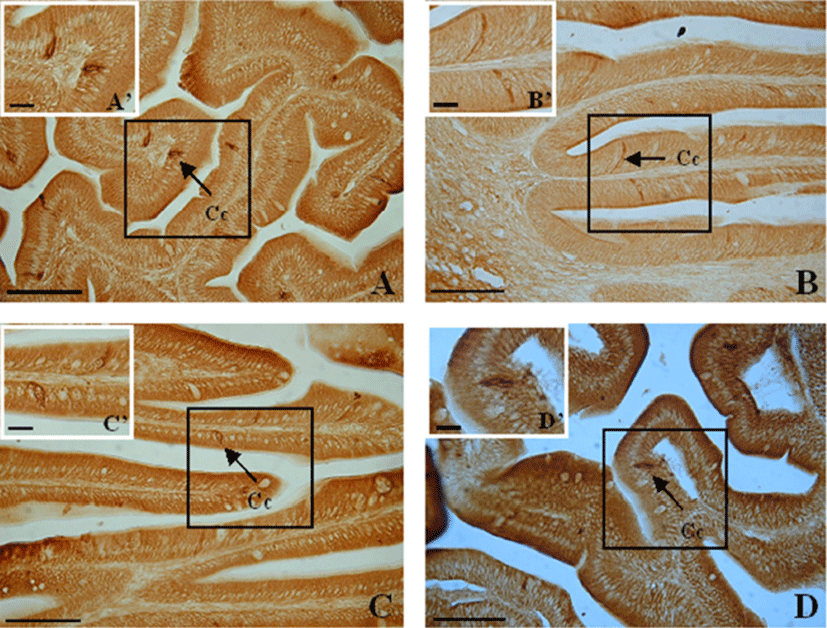
CCK-producing cells were observed from the anterior to the mid intestine portion. The numbers of CCK-producing cells were recorded from anterior intestine portion (175±5), mid intestine portion (38±26) (Table 1). Thus, from the anterior intestine portion to the mid intestine portion, the frequency of CCK-producing cells rapidly decreased (P<0.05).
Spindle-shaped cells and a few spherical and oval cells were dispersed among the epithelial cells of the mucosal folds. CCK-producing cells extended from the epithelium to the lumen of the intestine, with their thin apexes pointing toward the lumen (Fig. 3).
CCK-producing cells were observed in the anterior and mid intestine portion. The numbers of CCK-producing cells were recorded from anterior intestine portion (26±15), mid intestine portion (23±10) (Table 1). Thus, CCK-producing cells were scattered throughout the anterior and mid intestine portion.
Spindle shaped cells and a few spherical cells were dispersed among the epithelial cells of the mucosal folds (Fig. 4), but the frequencies were less than in S. geneionema and C. gulosus.
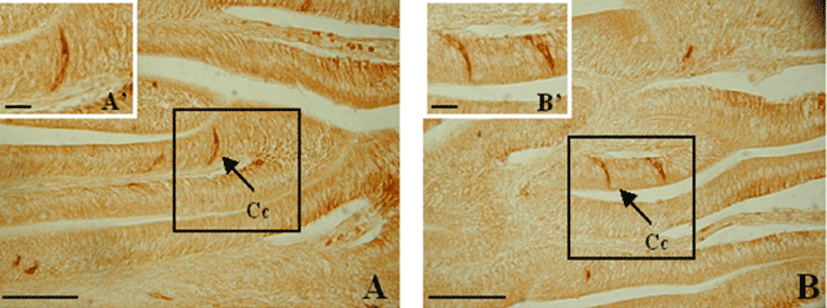
CCK-producing cells were observed only in the anterior and mid intestine portion. The numbers of CCK-producing cells were recorded from anterior intestine portion (83±23), mid intestine portion (10±4) (Table 1). Thus, compared to the anterior intestine portion, the frequency of CCK- producing cells in the mid intestine portion was greatly decreased (P<0.05).
Spindle cells were dispersed among the epithelial cells of the mucosal folds (Fig. 5). CCK- producing cells extended from the epithelium to the lumen but were less abundant than in S. geneionema and C. gulosus.
According to the location of the digestive tract and situation in that region, different regional distributions and relative frequencies of goblet cells were observed. These differences are shown in Table 2.
| Numbers of goblet cells/tissue section | ||||
|---|---|---|---|---|
| S. geneionema | C. gulosus | T. obscrus | R. giurinus | |
| Esophagus | 42±26c | 1,292±95a | 994±154b | 920±17a |
| Stomach | · | N.D. | N.D. | N.D. |
| Anterior intestine | 197±28b | 873±126b | 310±57c | 641±107b |
| Mid intestine | 271±48ab | 1,290±129a | 1,402±131a | 510±42bc |
| Posterior intestine | 339±26a | 784±49bc | 405±135c | 397±101cd |
| Rectum | 250±8ab | 446±51c | 368±41c | 287±55d |
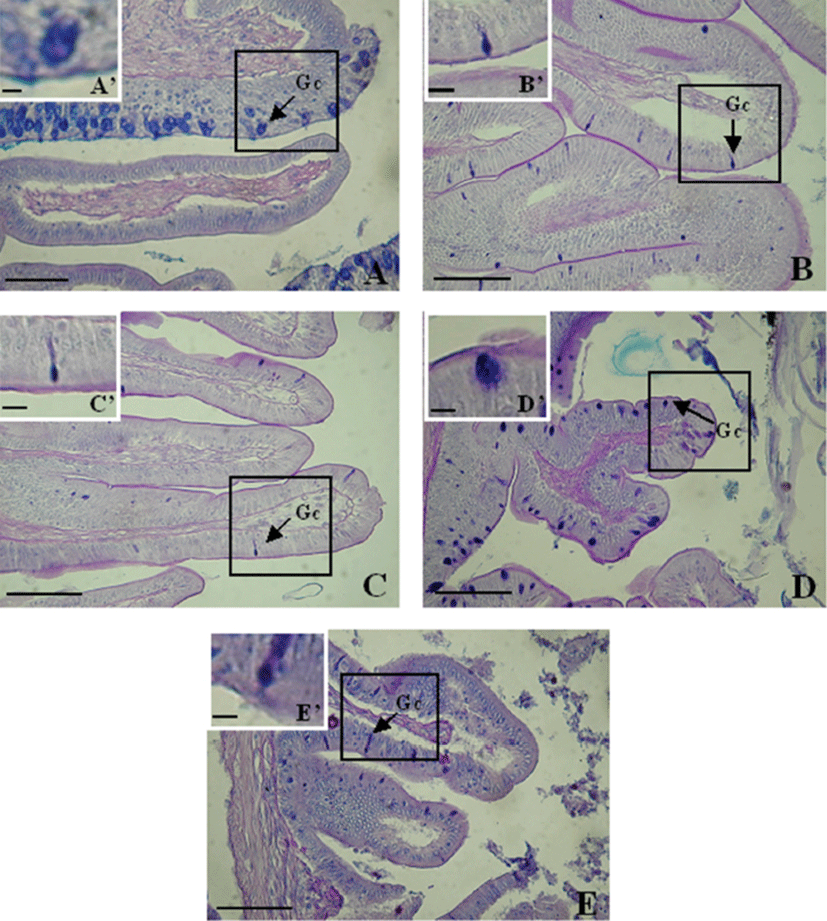
Goblet cells of this fish were distributed throughout the entire digestive tract. Mucus-secreting goblet cells were detected in the esophagus (42±26), anterior intestine portion (197±28), mid intestine portion (271±48), posterior intestine portion (339±26) and rectum (250±8) (Table 2). The greater abundance in the posterior intestine portion than in other portions of the digestive tract was significant (P<0.05).
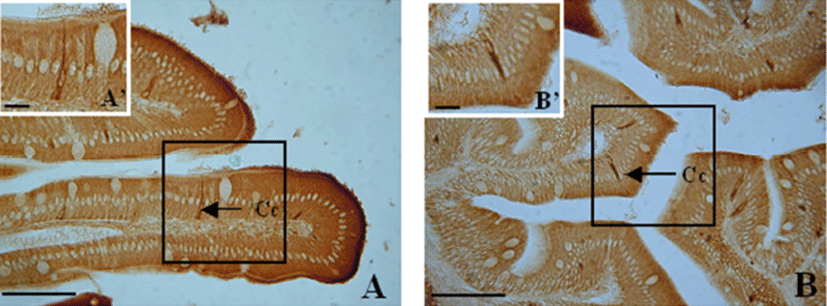
The goblet cells in the esophagus were mainly distributed within the epithelium and were spherical and oval (Fig. 6A). Toward the posterior intestine portion, these cells were densely distributed and mainly oval (Fig. 6).
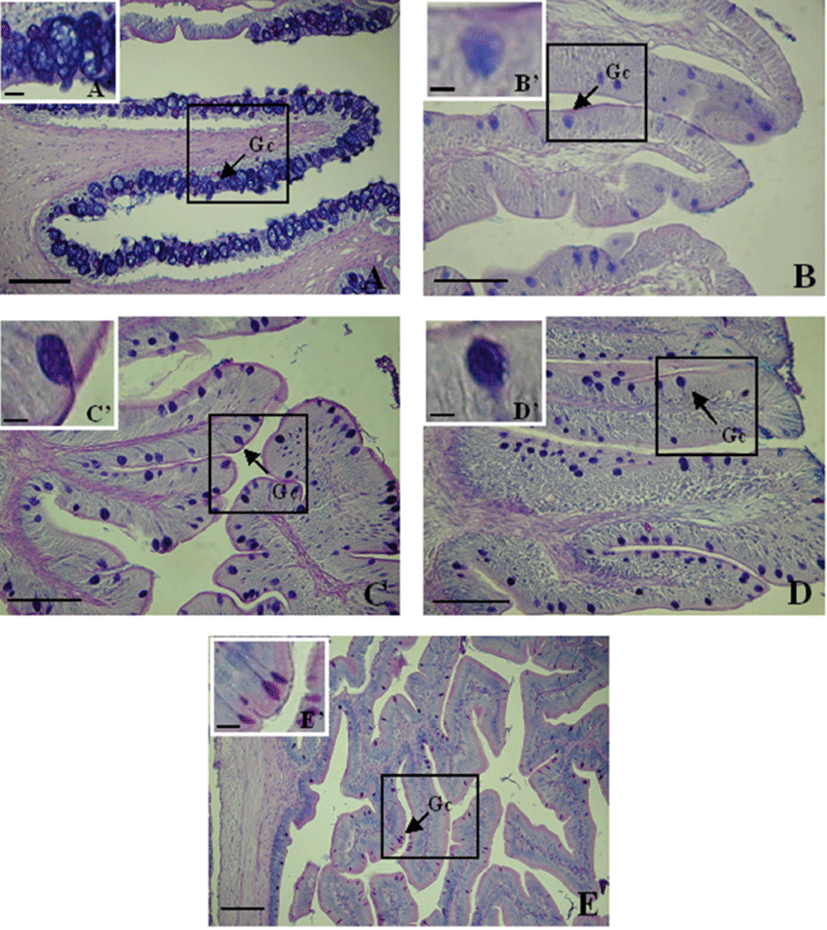
Goblet cells were not detected in the stomach of C. gulosus. Mucus-secreting goblet cells were detected in the esophagus (1,292±95), anterior intestine portion (873±126), mid intestine portion (1,290±129), posterior intestine portion (784±49), and rectum (446±51) (Table 2). Thus, these cells were significantly more abundant in the esophagus and mid intestine portion (P<0.05) and decreased toward the rectum.
The goblet cells in the esophagus were mostly located in the epithelia of the mucosal folds, and were large and spherical (Fig. 7A). In the other portions of the digestive tract, goblet cells were irregularly distributed from the upper to the lower parts of the mucosal folds (Fig. 7).
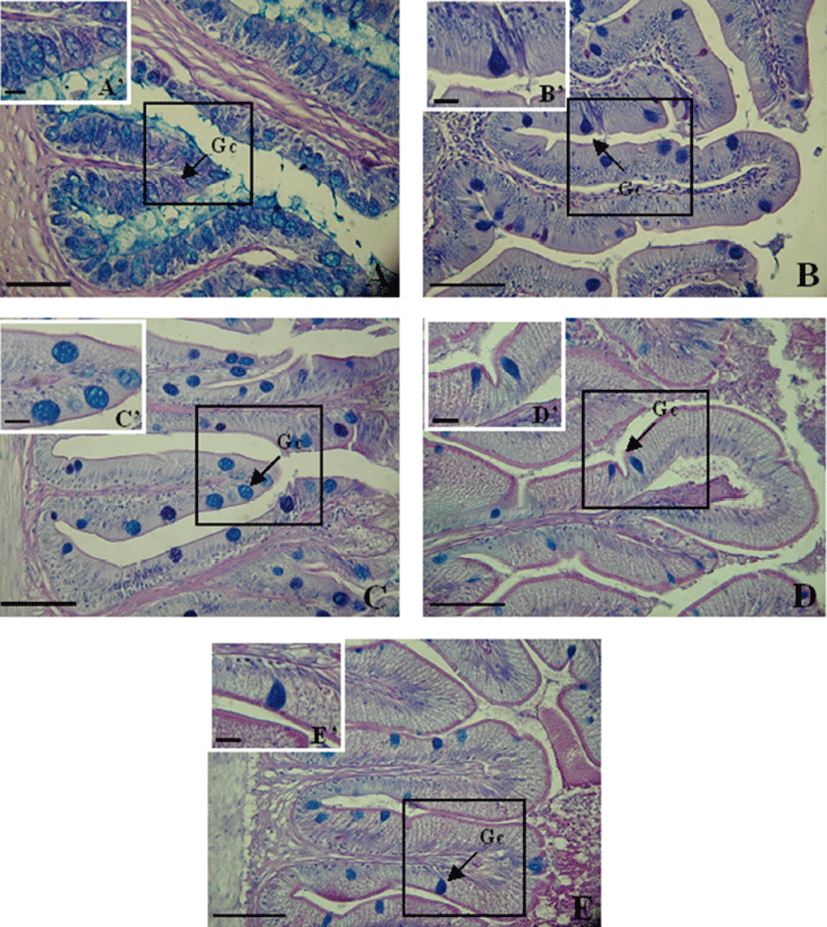
Goblet cells were not detected in the stomach of the trident goby. Mucus-secreting goblet cells were detected in the esophagus (994±154), anterior intestine portion (310±57), mid intestine portion (1,402±131), posterior intestine portion (405±135), and rectum (368±41) (Table 2). They were significantly more abundant in the mid intestine portion (P<0.05) and decreased in number toward the rectum.
The goblet cells in the esophagus were densely distributed within the epithelium of the mucosal folds and were mainly large and oval (Fig. 8A). In the other portions of the digestive tract, the cells were irregularly scattered from the upper to the lower parts of the mucosal folds and were spherical and oval (Fig. 8).

Goblet cells were not detected in the stomach of the giurine goby. Mucus-secreting goblet cells were detected in the esophagus (920±17), anterior intestine portion (641± 107), mid intestine portion (510±42), posterior intestine portion (379±101), and rectum (287±55) (Table 3). They were significantly more abundant in the esophagus (P<0.05). The number of goblet cells decreased toward the rectum.
The goblet cells in the esophagus were densely distributed and filled the lumen. They were large and spherical or oval (Fig. 9A). From the anterior intestine portion to the mid intestine portion, the cells were mainly spherical and were distributed throughout the mucosal folds. From the posterior intestine portion to the rectum, mainly oval shaped goblet cells were observed; these were also distributed throughout the mucosal folds (Fig. 9).
DISCUSSION
The digestive tracts of different fish species vary in morphology and structure. Analyses of the digestive systems of several species of fish have been conducted, including mummichog, Fundulus heteroclitus (Babkin & Bowie, 1928) flounder, Platichthys flesus (Jenkins et al., 1992) and Nile tilapia, Oreochromis niloticus (Morrison & Wright, 1999). Few studies have investigated the families Atherinidae, Blenniidae, Cobitidae, Cyprinidae, Gobiidae, Mugilidae, and Scaridae. In addition, the digestion and absorption properties of the digestive tracts of several stomachless fish belonging to the family Labridae have been studied (Ishida, 1936; Barrington, 1942; Al-Hussaini, 1947, 1949; Szarski, 1965; Chao, 1973).
The digestive tracts of most teleosts consist of an oral cavity, pharynx, stomach, intestine, rectum, and anus. Some Cypinidae and Gobiidae lack a stomach and pyloric ceca (Tanaka, 1969). Previously, we histologically examined S. geneionema, which has neither a stomach nor pyloric ceca, and C. gulosus, T. obscurus, and R. giurinus, which also do not have pyloric ceca (Hur et al., 2015).
The goblet cells in the fish digestive tract produce a lubricant for the mucosal surface, which prevents the mucous membrane from being damaged by physical or chemical action and protects it from the actions of digestive enzymes (Allen et al., 1986). Goblet cells in the digestive tracts of several species of fish have been studied with respect to mucus secretion: Konosirus panctatus, Carassius carassius, Parasilurus asotus, Thamnaconus modestus, and P. majors (Jo et al., 1984). The histochemical characteristics of intestinal mucus-secreting cells have been analyzed in Acanthopagrus schlegeii, Erosa erosa, and Sebastes inermis (Byeon & Jo, 1985), as well as in Pleuronichthys cornutus, Paralichthys olivaceus, Acanthogobius hasta, Zoarces gillii, and Lagocephalus wheeleri (Choi, 1996). According to these studies, the shapes and sizes of the goblet cells in the digestive tract depend on the species of fish and the intestinal locations of the cells. The amounts of mucin also depend on the species of fish and the particular region of the digestive tract, with the number of goblet cells generally increasing posteriorly (Reifel & Travill, 1979). Lee & Chin (1995) reported that the number of goblet cells of D. temmincki, a stomachless fish, increased toward the posterior portion of the intestine and the rectum. Similarly in S. geneionema, which is also a stomachless fish, the numbers of AB-PAS positive goblet cells increased from the anterior toward the posterior portion. In terms of the shape of these cells, the front and middle basal parts are slim and the long back part is oval and rounded. In C. gulosus, the goblet cells, which are ABPAS positive, are most abundant and most densely arranged in the mid intestine, and their shape ranges from largely oval to round. Like C. gulosus, the goblet cells of T. obscurus are AB-PAS positive and are distributed mostly in the mid intestine; the number of cells decreases toward the rectum. The cells are small and oval. In contrast to the goblet cells of S. geneionema, those of R. giurinus are distributed mostly in the vicinity of the esophagus and decrease in number toward the rectum. The cells are ABPAS positive and are rounded and oval. Thus, it appears that the distribution of goblet cells and their various forms depends on the type of food consumed and the species of fish; however, further and more detailed studies regarding the physiological mechanisms are needed.
In S. geneionema and C. gulosus, which live in seawater, their digestive action occurs in the posterior intestine portion and the mid intestine portion to protected and functions to activate digestion. In contrast, in T. obscurus and R. giurinus, which are brackish water fish, their digestive action occurs in the mid intestine portion and the anterior portion to protect and functions to activate digestion. Further studies of the modes of food ingestion by these fish, the contents of their digestive tracts, and the staining characteristics of the goblet cells need to be carried out.
In fish, several intestinal peptide hormones have been identified, mainly by means of immunohistochemical techniques (Fritsch et al., 1978; Noalillac-Depeyre & Hollande, 1981; Holmgren et al., 1982; El-Salhy, 1984a; El-Salhy, 1984b; Hansen et al., 1987; Garcia-Hernandez et al., 1994; Reinecke et al., 1997; Kurokawa et al., 2000). Hormonal factors seem to play important roles in the intestine of vertebrates, including fish (Lebenthal & Lebenthal, 1999). Cholecystokinin (CCK) is a major hormone controlling digestion and participates in the regulation of food intake and satiety (Peyon et al., 1997), as well as having stimulatory effects on pancreatic secretion (Bell, 1979; Biederbick & Elsässer, 1998). CCK is produced by endocrine cells scattered among the epithelial cells lining the intestine and, upon exposure to the appropriate food signals in the intestinal lumen, is released into the circulation (Liddle, 1997). In teleosts, CCK has been shown to retard gastric emptying (Olsson et al., 1999), regulate the secretion of pancreatic enzymes, stimulate gall bladder contractions in vitro and in vivo, and regulate intestinal peristalsis (Aldman & Holmgren, 1987; Rajjo et al., 1988; Aldman et al., 1992; Einarsson et al., 1997). Most studies, however, have focused on the timing of CCK expression and its distribution pattern in other species of fish, e.g., olive flounder (P. olivaceus), Atlantic halibut (Hippoglossus hippoglossus), and bluefin tuna (Thunnus thynnus), whose CCK- producing cells are found only in the anterior midgut, or in the early life stages of teleosts (Kurogawa et al., 2000; Kamisaka et al., 2001; Kamisaka et al., 2002). In ayu, Plecoglossus altivelis, and Atlantic cod, Gadus morhua, CCK-producing cells are distributed as far as the posterior intestine (Jӧsson et al., 1987; Kamisaka et al., 2003). In our study, CCK-producing cells in the digestive tract of the Gobiidae were found to differ remarkably in their regional distributions and relative frequencies. In C. gulosus, T. obscurus, and R. giurinus, these cells were only observed in the anterior and mid intestine portion. This distribution pattern is quite similar to that of olive flounder, P. olivaceus, Atlantic halibut, H. hippoglossus, and bluefin tuna, T. thynnus. In contrast, in the stomachless teleost S. geneionema, CCK-producing cells were observed throughout the digestive tract. This is also the case in ayu, P. altivelis (Kamisaka et al., 2003). Furthermore, in S. geneionema, the highest frequency of CCK-producing cells was observed in the anterior intestine, with a small number of cells distributed as far as the rectum. This result suggests that food digested by acid in the stomach moves on and is then delayed in the anterior and mid intestine, thereby ensuring effective stimulation of the CCK-producing cells. Also, the distribution pattern of CCK-producing cells closely resembles that of goblet cells. In stomachless teleosts, ingested food easily passes through the digestive tract until it reaches the posterior intestine portion. The distribution pattern of CCK-producing cells in S. geneionema, for example, seems to be well adapted to promoting optimal control of the digestive process. Our study suggests that CCK-producing cells are distributed effectively to make up for the effects of the lack of a stomach on the digestive process.
