INTRODUCTION
The dorsal surface of tongue contains numerous macroscopic papillae. These papillae differ somewhat in shape, are named according to their morphologic characteristics, and serve either a mechanical or a gustatory function (Davies et al., 1979; Boshell et al., 1982; Chamorro et al., 1986; Holland et al., 1989; Kumar and Bate, 2004). Me-chanical papillae were able to divide it into filiform, conical and lentiform papillae, and gustatory papillae were divided into fungiform, vallate and foliate papillae (Witt and Reutter, 1997). Goats have three different types of mechanical papillae and two different types of gustatory papillae according to their shape (Lee et al., 1996; Kumar et al., 1998).
Scanning electron microscopic studies of the tongue papillae in various animals have been reported for rodents (Iwasaki et al., 1996; Iwasaki et al., 1997), carnivore (Boshell et al., 1982; Holland et al., 1989; Ojima, 2001), ruminants (Davies et al., 1979; Emura et al., 2000; Eerdunchaolu et al., 2001), pigs (Kumar and Bate, 2004), horses (Chamorro et al., 1986; Pfeiffer et al., 2000), and various wild animals (Krause and Cutts., 1982; Furubayashi et al., 1989; Emura et al., 2004). A few studies have been performed with goats, examining their filiform, fungiform, conical, vallate, and lentiform papillae (Lee et al., 1996; Kumar et al., 1998). However, much less has been written about the morphological characteristics of the developing tongue in goats (Kim et al., 2012). Therefore, to understand the progress of developing vallate papillae (VP) in goats, we investigated the features of the VP on the dorsal surface of the tongue during development as shown by scanning electron microscopy.
MATERIALS AND METHODS
The tongues from three fetuses, four neonates and six juveniles of Korean native goat were used in each group. To investigate the morphological characteristics of the tongue during prenatal development, the tongues examined in this study were removed from 60, 90, 120, 150-day-old fetuses (neonates) originated from Korean native goats aged 2 to 4 years old with a body weight ranging from 23 to 33 kg by caesarean section and performed under general anesthesia using xylazine hydrochloride (10 mg/kg, i.v.; Bayer Korea Ltd.). The VP of the tongues were examined for morphological features. We also removed the tongues from goats at six different postnatal stages: on days 30, 60, 90, 120, 150, and 180, respectively. All animal experiments were performed according to the protocol set out in the guidelines of the Ethics Committee for Animal Experiments at Gyeongsang National University (Approval No. GNU-LA-10).
Tissues for scanning electron microscopy (SEM) studies were fixed using 2.5% glutaraldehyde 4 h at room temperature. Areas appropriate for inspection were dissected and osmicated in 1.0% osmium tetroxide at 4oC for 2 h. After washing with PBS three times, the tissues were dehydrated using a graded series of ethanol solutions. The specimens were prepared for critical drying point, and then placed on spinner stubs, and coated with gold to a depth of 100 µm in a SEM coating unit. The specimens were observed under a SEM (SEM-AL 300, Philips) operated at 15 KV.
The measurement of the VP from three to five different regions was performed by using a scanning electron microscope to measure the minimum and maximum diameters of the papillae of neonates to 180-day-old goats. We analyzed the statistical significance of differences in each group by using a two-tailed Student’s t-test (Prism; Graph Pad Software).
RESULTS
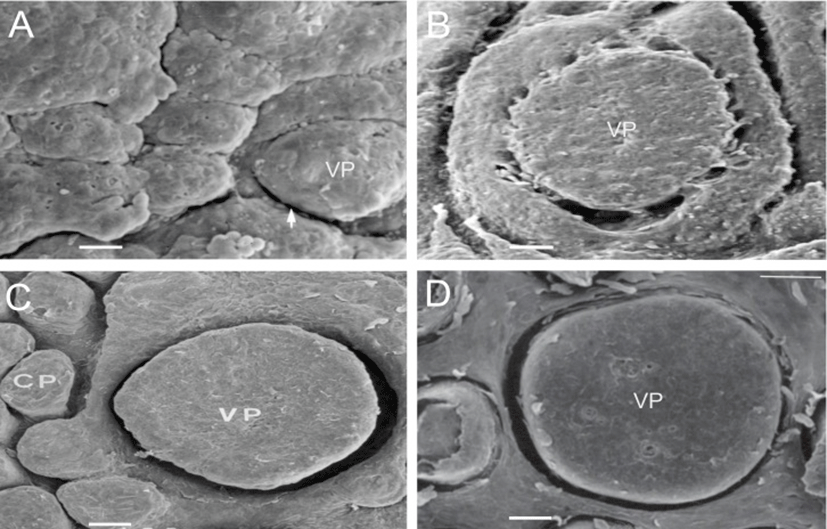
The VP of the goats were located on both sides of the midline around the tongue root during development. In prenatal development of the VP, round and low primordia of the VP was seen in 60-day-old fetuses (Fig. 1A). The moat of the VP was shallowly spread and still undifferentiated in 90-day-old fetuses (Fig. 1B). In 120-day-old fetuses, there was a deeply furrowed in the moat entirely surrounding the primordia of the VP (Fig. 1C); and the microridges and microplicae were well developed on the surface of the VP (Fig. 3A). In neonates, the VP were developed as complete as those of adults (Fig. 1D); and the developing taste pore was also observed on the epithelial surface of VP (Fig. 3B).
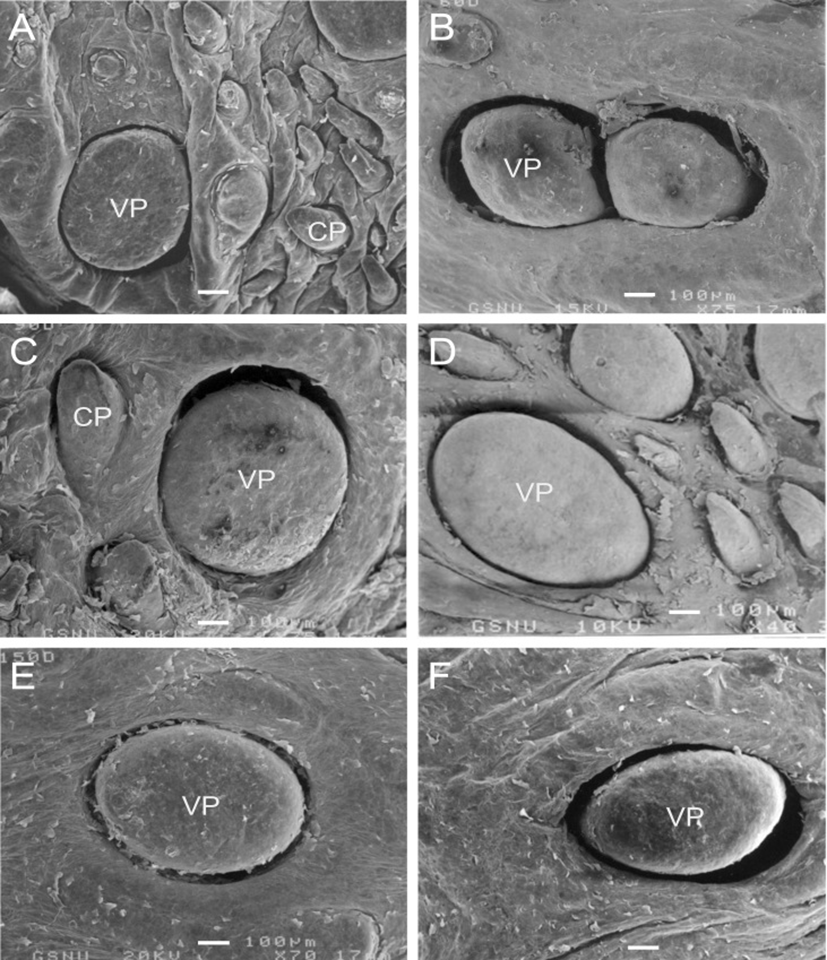
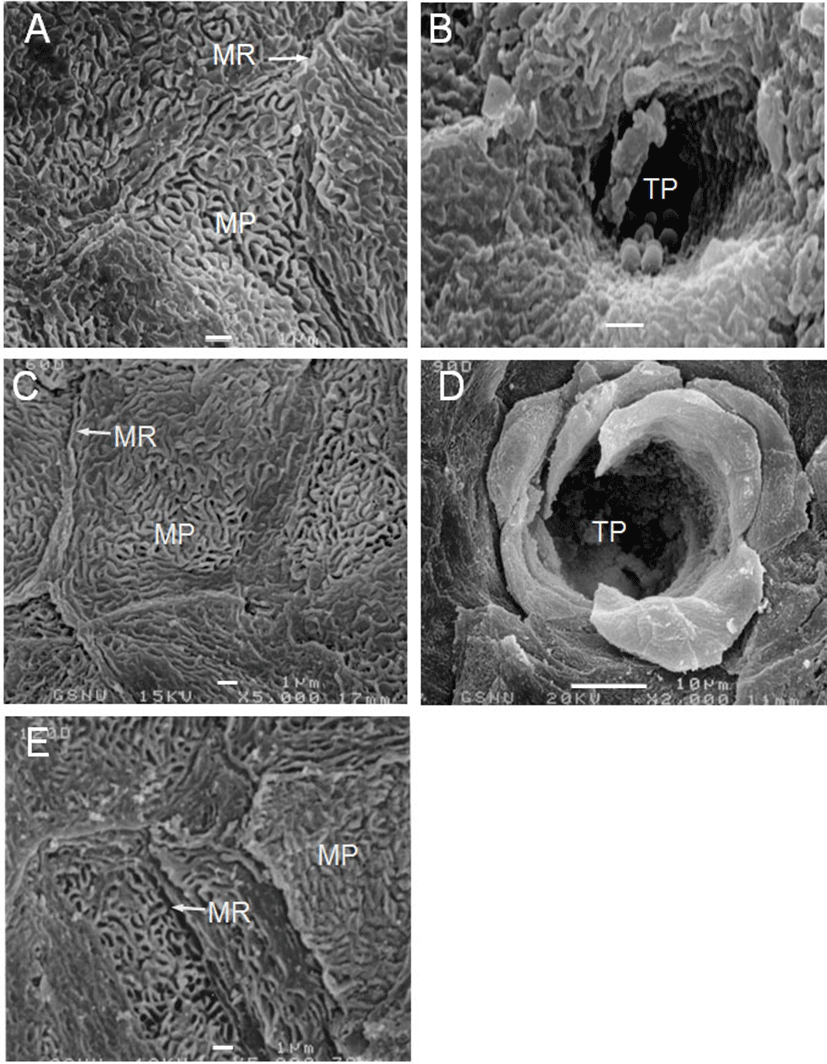
In postnatal development of the VP, the papillae were increased in size with slight morphological changes in postnatal day-30 (Fig. 2A). In postnatal day-60, twin VP were found to be surrounded by one primary papillary groove; and two VP that shared a moat was found (Fig. 2B). The microplicae and microridges on the epithelial surface of VP were thick and windingly round shape in postnatal day-60 (Fig. 3C).
In postnatal day-120 and -150, the VP were somewhat increased in size without much difference in shape (Figs. 2D, E); and the microplicae and microridges were very well developed on the epithelial surface in postnatal day-120 (Fig. 3E). In postnatal day-180, the moats of VP were more widely and deeply engraved than those of 150-day-old goats (Fig. 2F).
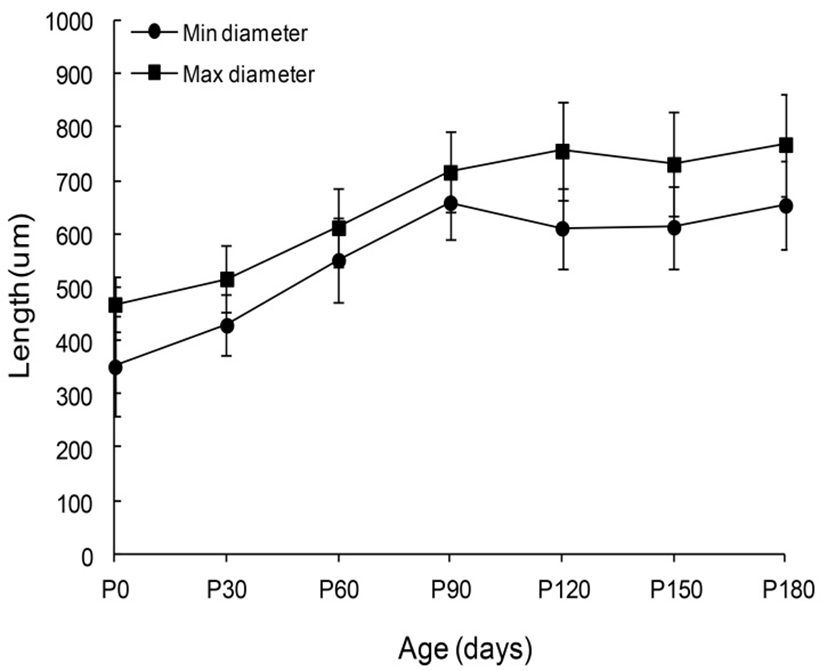
The sizes of the goat’s VP from neonates age to 180-days-old were shown in Fig. 4. The minimum and maximum diameters of newborn papillae were 351.0±94.0 and 468.0±53.0 µm respectively. Comparatively, the papillae diameters of 30-day-old goats were 429.0±57.0 and 516.0 ±63.0 µm. Those of 60-day-old goats were 551.0±79.0 and 612.0±73.0 µm. Those of 90-day-old goats were 659.0± 68.0 and 716.0±75.0 µm. Those of 120-day-old goats were 611.0±75.0 and 756.0±91.0 µm. Those of 150-day-old goats were 613.0±78.0 and 731.0±97.0 µm. Lastly the pa-pillae diameters of 180-day-old goats were 654.0±84.0 and 768.0±96.0 µm, respectively. However, no statistically significant differences were found between each age during the next 30 days of life.
DISCUSSION
There are many reports based on scanning electron microscopy of the three-dimensional structures of the lingual papillae in mammals (Davies et al., 1979; Boshell et al., 1982; Chamorro et al., 1986; Holland et al., 1989; Kumar and Bate., 1998). Kumar et al. (1998) have studied the tongue for filiform, fungiform, conical, vallate and lentiform papillae on the dorsal surface of the goat tongue. Lee et al. (1996) also performed scanning electron microscopic studies of the tongue. However, no comparative studies on changes in the three-dimensional structure of the VP during the development of goats using SEM have been reported.
Concerning the development of lingual papillae, gustatory papillae grow faster than mechanical papillae in humans (Chunhabundit et al., 1992). In mice and rats, the primordia of filiform papillae are formed after the appearance of the rudiments of fungiform and VP (Iwasaki et al., 1996; Iwasaki et al., 1997). On the other hand, the primordia of conical papillae remain in a less-developed form in 90-day-old goat fetuses (Kim et al., 2012). In this study, however, the primordia of VP were found to be better developed than those of the conical papillae in 90-day-old goat fetuses. Therefore, our finding that gustatory papillae were developed prior than mechanical papillae followed the same trend that has been shown to be the case in rodents and humans.
During development, the conical papillae of the mechanical papillae had been found to have an obliquely-sectioned cylindrical shape with an empty inside in 50 day-old goats; and they were observed to have a completely compacted shape on inner surface in 60 day-old goats (Kim et al., 2012). In this study, however, the VP of the gustatory papillae were had a round or an oval shape during development; and their papillae in neonates appear to be shaped similar to those of adults. Therefore, these results confirmed that the mechanism governing morphogenesis and the development of gustatory papillae might be differ-rent from that of mechanical papillae.
The morphology of the VP in the newborn mouse had been found to have central papillae and flanking papillae, which formed a horseshoe-like structure around the central papillae (Iwasaki et al., 1996). The tongue of the tree shrew had been found to have pin-cone-like papillae having small and many pins (Chunhabundit et al., 1992). Opossum tongue had been found to have a typical vallate-like shape surrounded by a deep moat with small secondary papillae observed on the tips of the VP (Krause and Cutts., 1982). However, a flattened-oval-like shape and secondary papillae were not observed in ruminants (Lee et al., 1996; El Sharaby et al., 2012), they were not observed in the present study of developing goats. On the contrary, complex VP were sometimes spotted on the tongues of adult Korean native goats (Lee et al., 1996). Similarly, two fused VP were also observed in this study.
According to the morphology of the epithelial surface, the surface structure of the lingual epithelia of 12-day-old rat fetuses and those of 15-day-old mouse fetuses had been found to be very similar (Iwasaki et al., 1996; Iwasaki et al., 1997). In this study, the microridges in 120-day-old fetuses were compactly sticking up on the surface of VP. Likewise, well-developed microplicae on the epithelial surface of lingual papillae having similar surface structures were observed in 120-day-old goats. Therefore, the surface structures of the lingual epithelia in Korean native goats were very similar to those of other species.
Concerning the size of the lingual papillae of Korean native goats, Lee et al (1996) reported that, for Korean native goats weighing approximately 15 kg, the VP were round or oval in shape and their diameter ranging from 500 to 850 µm with the average number of VP being 34.8. In this study, papillae sizes were 351~468 µm in diameter among newborns, 551~612 µm in diameter among 60-day-old goats during the weaning period, and 654~768 µm in the maturation period of 180-day-old goats. Although the size of the VP in adult goats was similar to those of other studies, that size gradually increased until the 90-day-old goat during development. However, conical papillae belonging to mechanical papillae were still increased in size even after 120 days following birth (Kim et al., 2012). Taken together, these findings indicate that the VP of goats manifest in a variety of shape and size during both prenatal and postnatal development.
