INTRODUCTION
Human mesenchymal stem cells (hMSCs) show promise in the field of regenerative medicine because they can modulate numerous incurable diseases. Mesenchymal stem cells, also called mesenchymal stromal cells, are a type of adult stem cell, that play a role in maintaining and repairing various adult tissues and organs (Pittenger et al., 1999). hMSCs have emerged as a promising candidate for the cell-based therapy of various diseases, such as cardiovascular diseases (Faiella & Atoui, 2016), diabetic nephropathy (Liu & Tang, 2016), and diverse brain injuries including stroke, neural trauma, and heatstroke (Hsuan et al, 2016).
MSCs are non-hematopoietic, multipotent cells that are present in adult marrow. They are capable of self-renewal and multilineal differentiation into various tissues of mesodermal origin, such as bone, cartilage, tendon, fat, heart, muscle, and marrow stroma (Pittenger et al., 1999); (Deans & Moseley, 2000). hMSCs show several superior properties for therapeutic use compared to other types of stem cells. For successful cell-based therapies with hMSCs, a substantial number of cells are needed, requiring extensive ex vivo cell expansion. Owing to prolonged ex vivo expansion needed in the clinic to obtain a sufficient number of cells for therapy, long-term culture will likely evoke continuous changes in hMSCs, including cellular senescence (Yang et al., 2012); (Park et al., 2005). Considering the strengths and weaknesses of hMSCs in ex vivo cultures would provide us with some novel approaches for overcoming limitations to their therapeutic efficacy and maximize their clinical value.
Advantages of MSCs over Other Stem Cell Types in Clinical Applications
Among various stem cell types, hMSCs show several superior properties for clinical use in cell-based therapies. The benefits and limitations of each stem cell type are discussed and summarized in Table 1.
Embryonic stem cells (ESCs) are produced in the inner cell mass of the blastocyst during mammalian embryonic development, late in the first week after fertilization (Evans & Kaufman, 1981); (Boyle et al., 2006). They are considered pluripotent, and can give rise to the three embryonic germ cell layers, and almost all types of cells found in an organism. Because of their pluripotency, they have attracted much attention. Some pluripotent human
ESC lines are established by using cells obtained from the inner cell mass of an early-stage human embryo (Thomson et al., 1998). Many protocols have been established for the differentiation of human ESCs into numerous mature and functional types of cells (Lee et al., 2007). Nevertheless, broad clinical application of ESCs remains controversial owing to concerns about teratoma formation and ethical issues raised from the embryonic source of the tissues (Wang et al., 2016).
Ethical controversies regarding ESCs led to the development of induced pluripotent stem cells (iPSCs), this development was recognized by Nobel Prize in Medicine in 2012, only six years after its initial publication. iPSCs were first reprogrammed from terminally differentiated fibroblasts by the transduction of four defined transcription factors, such as Oct3/4, Sox2, c-Myc and Klf4 or Nanog or Lin28 (Takahashi & Yamanaka, 2006); (Takahashi et al., 2007); (Zhang et al., 2016). Like ESCs, iPSC also show great pluripotency. Recently, several promising protocols have been developed for differentiating human iPSCs into various types of cells (Tian et al., 2015); (Xia et al., 2013); (Carpenter et al., 2012). Even though iPSCs are attractive candidates for cell-based therapy, their use is limited by the associated risk of teratoma formation after transplantation, which is also a concern in ESC applications. Genomic instabilities and epigenetic variations of iPSs, such as aneuploidy (Amps et al., 2011), subchromosomal copy number variations (Laurent et al., 2011); (Martins-Taylor et al., 2011); (Mayshar et al., 2010), single nucleotide variations (Cheung et al., 2011); (Young et al., 2012), variations in X Chromosome inactivation (Wutz et al., 2012), and aberrant DNA methylation (Nazor et al., 2012), have been reported. These variations exist between iPSC lines, between iPSC and ESC lines, between different passages of the same iPSC lines, and even between different populations at a specific passage of the same iPSC line. Such variations potentially affect the properties of iPSCs and undermine their utility in cell-based regenerative medicine (Liang & Zhang, 2013).
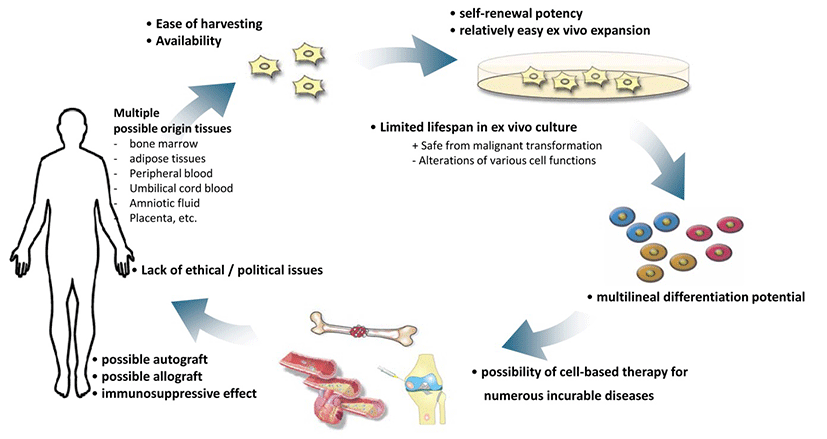
MSCs afford several advantages for clinical use, such as availability and ease of harvesting; multilineal differentiation potential; potent immunosuppressive effects; safety without any possibility of malignant transformation after infusion of allogeneic cells, which is common in the case of ESCs and iPSCs; and the lack of ethical issues that occur with the application of human ESCs. One of the most promising benefits of MSCs for cell-based therapy is their availability and ease of harvesting. MSCs can be isolated and expanded from the stroma of virtually all organs such as bone morrow, adipose tissue (Zuk et al., 2002), umbilical cord blood (Romanov et al., 2003), peripheral blood (Chong et al., 2012), amniotic fluid (In 't Anker et al., 2003) and placenta (In 't Anker et al., 2004). The most preferred and abundant sources are bone marrow and subcutaneous adipose tissue (Crisan et al., 2008); (Turinetto et al., 2016). Upon isolation, hMSCs are characterized by their ability to adhere to the surface of plastic plates and fibroblast-like morphology. Their overall isolation procedure is relatively simple compared to that for other types of stem cells (Pittenger et al., 1999). Isolated and expanded ex vivo cells can be differentiated into osteocytes, chondrocytes, adipocytes, myocytes, and marrow stroma (Pittenger & Martin, 2004). Another characteristics of hMSCs that contributes to their therapeutic effect is that they secrete various soluble growth factors and cytokines that act in endocrine and paracrine fashions, in turn affecting their therapeutic effect (Monsel et al., 2015).
Rodent and human MSCs are advantageous owing to their immunomodulatory characteristics in both in vitro and in vivo transplant models, allowing them to act as a universal reserve of donor cells (Atoui & Chiu, 2012). MSCs’ unique immunotolerant phenotype is due to their special distribution of surface markers that allows them to escape detection from immune cells. They possess low levels of MHC class I, CD40, CD80, and CD86, with no MHC class II molecules (Pittenger et al., 1999); (LeBlanc et al., 2003); (Faiella & Atoui, 2016). This immunomodulatory phenotype of MSCs permits the potential to use allogeneic cells for patients.
MSCs have limited ability to proliferate in cultures. Senescence activations were observed in hMSCs from different sources, such as bone marrow (Park et al., 2005); (Minieri et al, 2015), dental pulp (Muthna et al., 2010), cord blood (Ko et al., 2012), and endometrium (Burova et al., 2013). The limited replicative lifespan of MSCs guarantees safety from the threat of malignant transformation after transplantation. However, this limited cell lifespan can be a double-edged sword for clinical applications of hMSCs, as discussed later.
Fig. 1 summarizes all the advantageous characteristics of hMSCs.
Prerequisites for Successful Cell Therapy with MSCs
For successful cell-based therapies, stem cells must be able to differentiate into specific targeting cells, or must act via paracrine mechanisms. Their extraction and isolation must be feasible and transplantation into humans must be safe and effective. Furthermore, to maximize the therapeutic effects of cell-based therapy, a substantial number of cells is essential, requiring extensive ex vivo cell expansion for most cell types (Faiella & Atoui, 2016). The use of hMSCs in regenerative medicine strategies based on cell therapy relies on the ability of MSCs to proliferate readily and produce differentiated cells that can substitute for the targeting affected tissue. Therefore, it should be considered whether, after the ex vivo expansion necessary before their therapeutic use and transplantation, these cells still possess the properties of stem cells namely, self-renewal and multilineal differentiation.
Factors that Limit the Stemness of MSCs: Cellular Senescence
Although MSCs have been widely applied in cell-based therapy, their clinical usefulness remains limited. MSCs from different donors are heterogeneous. Cell passages and culture conditions in vitro affect the cell phenotype (Liu and Tang, 2016); (Turinetto et al., 2016); (Kretlow et al., 2008). Furthermore, MSC’s cellular senescence significantly impairs their proliferation and differentiation potential (Park et al., 2005); (Turinetto et al., 2016). Aging affects the cell subpopulation dynamics and diminishes the function of MSCs (Duscher et al., 2014); (Wang & Ren, 2014). The use of hMSCs is expected to be useful for treating degenerative diseases in elderly populations; however, the limited potential of the MSCs of aged patients can limit the efficacy of an autologous cell-based therapeutic approach.
Cellular senescence aggravates various functions of hMSCs. With senescence, hMSCs show decreased differentiation potential and altered commitment between osteogenic and adipogenic lineage determination, although the direction of this shift remains controversial. Some studies reported that the osteogenic activity of hMSCs were reduced according to their ex vivo culture (Banfi et al., 2000). On the other hand, the osteogenic potential of senescent hMSCs remained the same or even increased in some studies (Wagner et al., 2008); (Bruder et al., 1997); (Digirolamo et al., 1999). Some studies reported that senescent hMSCs reveal the impaired balance between osteogenic differentiation to the osteogenic versus adipogenic lineages (Kim et al., 2012).
The altered immunoregulatory activities of hMSCs during cellular senescence also influence the therapeutic potentials of cells (Sepulveda et al., 2014). Studies related to the altered immunomodulatory functions of senescent hMSCs strongly support the idea that in vivo administration of senescent hMSCs could evoke an inflammatory response at a systemic level and lead to sepsis (Turinetto et al., 2016).
Proper cell migration toward relevant stimuli is also an essential factor for the functional engraftment of hMSCs into diseased loci. Another study demonstrated altered cell migration due to changes in MSC surface markers during prolonged cultivation, which can diminish the homing ability of hMSCs (Jung et al., 2011).
Despite arrested growth upon senescence being a potent tumor suppressor mechanism, which can protect hMSCs from malignant transformation after transplantation, paradoxically, senescence itself can affect the paracrine factors secreted from cells to evoke the tumor-promoting function (Liu et al., 2007). These studies suggest that the senescence of hMSCs increases the complexity of paracrine communication among cells and further enhances their detrimental tumor-promoting effect.
Trials to Overcome Senescence of MSCs
Various approaches have been tested to expand the lifespan of hMSCs and maximize their clinical usefulness by improving their performance.
The ectopic expression of telomerase in hMSCs is one way to combat the replicative senescence of cells (Park et al., 2008); (Tang et al., 2013). However, this approach can be dangerous owing to the possibility of malignant transformation of cells. Therefore, gene engineering of telomerase in hMSCs should be avoided in clinical applications.
Allopathic treatments to solve the phenotype of senescence in hMSCs may also be employed. The decreased expression of histone deacetylases is one of the phenotypes of senescent MSCs; therefore, the use of a histone acetyltransferase inhibitor prevents the replicative senescence of MSCs (Jung et al., 2010). Additionally, rapamycin has been shown to reverse the senescent phenotype and improve immunoregulation (Gu et al., 2016).
Cellular senescence can be reduced by modulating the oxidative stress level because oxidative stress is one of the main causes of senescence. Various attempts have been made to reduce oxidative stress during the in vitro culture of hMSCs. Some studies suggested that MSCs cultured under hypoxic conditions during in vitro culture can escape from senescence and show prolonged lifespan (Fahrer et al., 2007); (Jin et al., 2010). Antioxidants can be another effective and safe alternative to overcome the senescence of hMSCs. Reducing oxidative stresses by adding anti-oxidants, such as ascorbic acids or N-acetylcysteine has been shown to prolong the replicative lifespan of human cells including hMSCs in vitro (Kashino et al., 2003); (Lin et al., 2005).
A separate attempt to maintain hMSC’s potential for self-renewal and differentiation by modifying the medium compositions showed limited success (Gharibi and Hughes, 2012). Medium supplementation with fibroblast growth factor-2, platelet-derived growth factor-BB, ascorbic acid, and epidermal growth factor increased the proliferation potential of cells and increased their lifespan. However, the differentiation potential could not be maintained with medium supplementation (Gharibi and Hughes, 2012).
Many trials are currently aiming to overcome the limited lifespan of hMSCs. This limitation is a double-edged sword for clinical applications. On the one hand, it solves the problem of malignancy; on the other hand, it limits various functions that mitigate the therapeutic effect, including self-renewal, multi-potency, migration, and immunomodulatory functions. Despite the need for more studies to improve the conditions of ex vivo expansion of cells, hMSCs are still considered a safe and effective source for cell-based therapy for numerous diseases.
