INTRODUCTION
Olive flounder (Paralichthys olivaceus), a flatfish species, is most important fish species for aquaculture in Asian countries, including Korea, Japan and China (Zheng & Sun, 2011). Olive flounder, which has a flattened oval body, occurs mainly in benthos at depths of 10 to 200 m and is widely distributed in the water surrounding Korea (Park et al., 2012). Olive flounder is cultivated mainly on the southern coast of Korea and Jeju Island, and its demand is increasing gradually (Kim et al., 2014).
Fishes are ideal subjects for developing cell lines as they are of considerable interest for basic studies in molecular, cellular and developmental biology as well as for commercial purposes. Second, fishes are characterized by high fecundity, large transparent embryos and rapid development (Alvarez et al., 2007). Cell lines are important for research in virology, immunology, genetics, oncology, developmental biology, toxicology, medicine, biotechnology, epidemiology, molecular carcinogenesis and functional genomics (Gomez-Lechon et al., 2008; Wang et al., 2010). Recent studies have evaluated chilling injury, cooling rates and cryoprotectant toxicity in various fish species (Suzuki et al., 1995; Zhang et al., 1995; Robertson, 1998).
The first fish cell line established was embryonic cells from rainbow trout (Oncorhynchus mykiss) (Wolf & Quimby, 1962), followed by Coho salmon (Oncorhynchus kisutch) (Ristow & De Avila, 1994), white bass (Morone chrysops) (Shimizu et al., 2003), sea perch (Lateolabrax japonicus) (Chen et al., 2003a), red sea bream (Chrysophrys major) (Chen et al., 2003b), haddock (Melanogrammus aeglefinus) (Bryson et al., 2006), Atlantic cod (Gadus morhua) (Holen et al., 2010), marin medaka (Oryzias dancena) (Lee et al., 2013), Honmoroko (Gnathopogon caerulescens) (Higaki et al., 2015) and Nile tilapia (Oreochromis niloticus) (Fan et al., 2017). To develop embryonic stem cell lines, a gene-targeting approach has been used in, for example, zebrafish (Danio rerio) and medaka (Oryzias latipes) (Hong et al., 1996; Chen et al., 2003a). Such cell lines can be used to produce knockout fish for in vivo analysis of gene function (Hong et al., 1996).
To date, several olive flounder cell lines have been developed: FG-9307 (flounder gill 9307) cells (Tong et al., 1998), Hirame natural embryonic cells (HINAE) (Kasai & Yoshimizu, 2001), flounder spleen and gill cells (Kang et al., 2003), flounder embryonic cells (Chen et al., 2004) and Paralichthys olivaceus brain cells (Zheng et al., 2015). It is important to establish various useful cell lines from olive flounder for basic research and biotechnological application.
Therefore, the purpose of this study is to develop new cell line that can be used for various research areas, instead of previous developed olive flounder cell lines. Here, a multipotent olive flounder cell line was developed by primary culture of embryonic cells. This cell line was characterized in terms of chromosomal abnormalities, growth, expression of pluripotency genes and transfection ability.
MATERIALS AND METHODS
Blastula-stage flounder embryos were harvested after 8 h in seawater at 18℃ post-fertilization and prepared for cell culture. For each culture, ~50–70 embryos were treated with antibiotics (1×), washed with DPBS (Gibco) and homogenized. The chorion membranes and cell debris were removed using the 40 μm cell strainer. The homogenate was centrifuged at 195×g for 15 min at 20℃, and single cells were harvested by gentle pipetting. After several washes with growth medium (GM), the cells were transferred to GM in a cell culture flask (surface area 25 cm2) (Corning).
Leibovitz’s L-15 complete GM (L-15, Gibco) supplemented with antibiotic-antimycotic (Gibco), fetal bovine serum (FBS, Gibco), flounder serum (FS), flounder embryo extract (EE).
For prepare the FS and EE, blood samples were collected from olive flounder and allowed to clot at 4℃ for 4 h. After centrifugation, the FS was collected into new flash tubes, heat-inactivated in a water bath at 56℃ for 30 min and subjected to membrane filtration (0.2 μm) to generate FS. Olive flounder embryos were washed with Dulbecco’s phosphate-buffered saline (DPBS; Gibco) supplemented with 4% antibiotics and homogenized on ice using a glass homogenizer. The homogenate was centrifuged at 1,750×g for 20 min at 4℃. The supernatant (EE) was collected, filter-sterilized and stored at −20℃ until use. FS and EE concentrations were determined by the Warburg-Christian assay using the NanoVue spectrophotometer (GE Healthcare) (data not shown).
Embryonic cell line which named OFEC-17FEN was cultured at 20℃ in an incubator, and the medium was changed every 2–3 days. Upon reaching 80% confluence, the cells were subcultured at a ratio of 1:2 according to a standard trypsinization method. Briefly, cells were washed twice with GM and dissociated in trypsin-ethylenediaminetetraacetic acid (EDTA) (Gibco) solution for 4 min at room temperature. The trypsin-EDTA solution was removed, and GM was added. For cryopreservation, cell cultures were suspended in 1 mL GM with 10% dimethyl sulfoxide (Sigma-Aldrich) and 50% FBS and then stored in isopropyl alcohol at −80℃.
Cells in GM were seeded at 3.5×104 per well into five wells of a 24-well plate (Corning). The cells were incubated for 8 days at 20℃, with a medium change every 3 days. Next, cells were suspended in trypsin-EDTA for 4 min, centrifuged for 5 min at 280×g at 20℃, and the trypsin-EDTA was replaced with GM (1 mL). Cells were counted daily using a hemocytometer (Sigma, Bright-Line). Doubling time was calculated using the linear part of the growth curve as follows: Doubling time=duration×Log(2)÷[Log(final conc.)–Log(initial conc.)].
OFEC-17FEN cells (21 passages) were used for chromosome analysis according to a previously published method (Wang et al., 2010) with slight modifications. Briefly, cells were treated with 1 μg/mL colchicine (Sigma) for 3 h at 24℃, then harvested by scraping the flask using a sterile cell scraper (SPL; 290 mm length, 20 mm blade) and suspended in 0.075 M KCl, then incubated for 20 min at room temperature. The KCl was removed, and 4 mL methanol: acetic acid (3:1) fixative solution were added gently but rapidly using a glass Pasteur pipette (Volac; 230 mm). The cells were incubated at room temperature for 30 min, 2×fixative solution was added, and the cells were dropped onto a fixative solution-treated slide glass and stained for 8 min with 8% Giemsa (Gibco). Finally, chromosomes of over 100 metaphase cells in OFEC-17FEN cells were visualized under microscope (Leica, DE/EM6000B) using oil immersion optics (Merck KGaA) at 1,000× magnification.
Expression of pluripotency genes was evaluated in OFEC-17FEN cells (11 passages) and in 4-, 8-, 16-, 32- and 64-cell-stage and morula-stage embryos. Total RNA was extracted from OFEC-17FEN cells using TRIzol(r) (Ambion) according to the manufacturer’s instructions. Total RNA was treated with DNase (Promega), and 0.5 μg was reverse transcribed into cDNA using the First-Strand cDNA Synthesis Kit (Roche) according to the manufacturer’s instructions.
Segments of the POU domain, class 5, transcription factor 1 (POU5f1), also known as sex determining region Y-box 2 (SOX2), NANOG, PR domain containing 14 (PRDM14), Krüppel-like factor 4 (KLF4), Sal-like protein 4 (SALL4) and 18S ribosomal RNA (18S) genes were amplified by reverse transcriptase polymerase chain reaction (RT-PCR) using PCR PreMix (AccuPower PCR Primix and Master Mix, Bioneer) according to the manufacturer’s instructions. Briefly, 1 μL template cDNA was mixed with 1 μL (10 pmol) each primer (Table 1), 7 μL distilled water and 10 μL PreMix. RT-PCR involved an initial denaturation step of 5 min at 95℃, followed by 30 cycles of denaturation for 30 s at 95℃, annealing for 30 s at 55℃ (60℃ for SOX2 and KLF4) and extension for 30 s at 72℃. The RT-PCR products were electrophoresed on a 1.5% agarose gel.
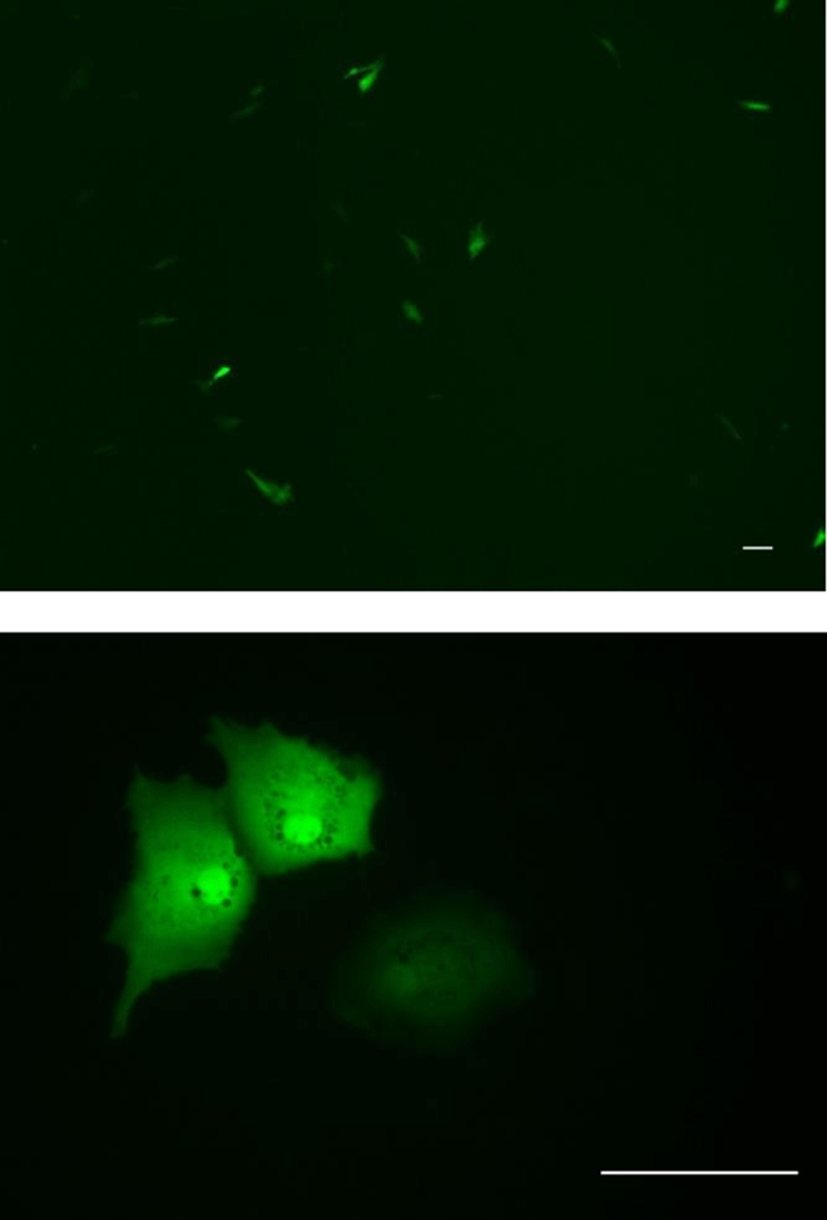
OFEC-17FEN cells (26 passages) were seeded at a density 5×104 per well in 24-well plates at 20℃. Cell monolayers (80% confluent) were transfected with pEGFP-c1 plasmid DNA and an expression plasmid using the JetPEI kit (Polyplus) according to the manufacturer’s instructions. Each well contained 1 μg 500 ng pEGFP-c1 plasmid DNA in 150 mM NaCl (final volume 50 μL) and 2 μL jetPEI reagent in 150 mM NaCl (final volume 50 μL). Green fluorescence signals were visualized under a fluorescence microscope (Carl Zeiss, Axio Vert A1) at 48 h post-transfection.
RESULTS
A new olive flounder (Paralichthys olivaceus) cell line which named OFEC-17FEN was developed by primary cell culture from the blastula stage of embryos. Cell line was cultured at 20℃ in incubator. Then first subculture was also accomplished. Morphologically, the OFEC-17FEN cell line was composed primarily of epithelial-like cells (Fig. 1). To date, OFEC-17FEN cells have been subcultured for >30 passages over ~200 days.
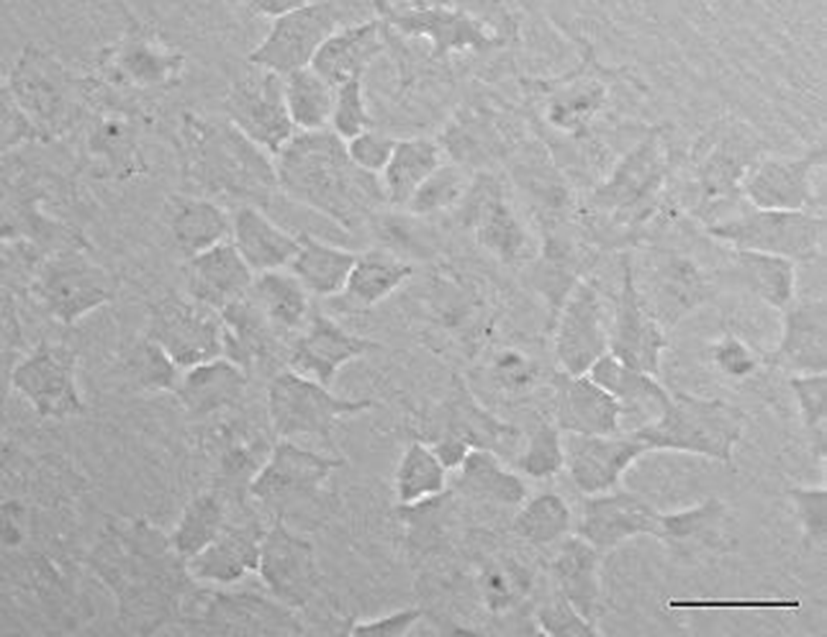
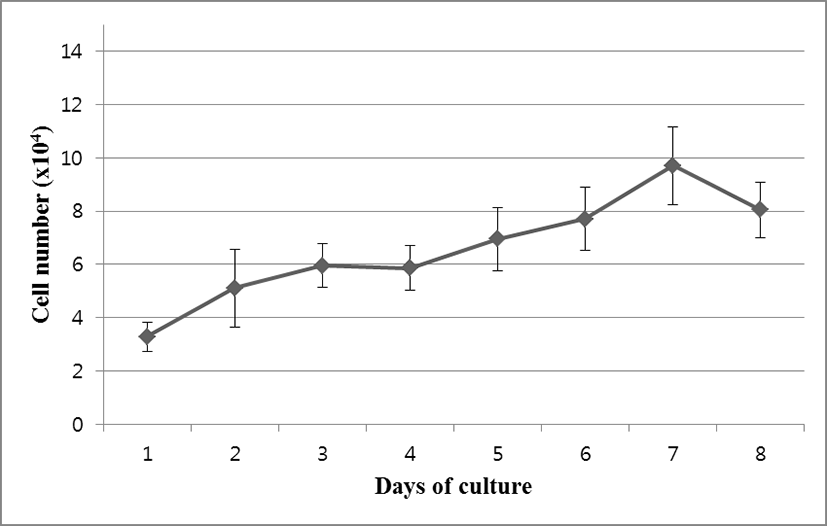
OFEC-17FEN cells were evenly attached to the surface of the well within the 24 hours after seeding. Cell growth was assessed by counting cell numbers with a hemocytometer. During the growth period, the number of the cells increased, and the cells grew to confluence. Cell growth performance is shown in Fig. 2. The OFEC-17FEN cell density was 3.29×104 /mL on day 1 and peaked at 9.7×104 /mL on day 7, with a doubling time of 114.34 h (Fig. 2). Growth curve showed a decrease in proliferation starting from day 8. Thus, the OFEC-17FEN cell line maximum density was reached at the day 7.
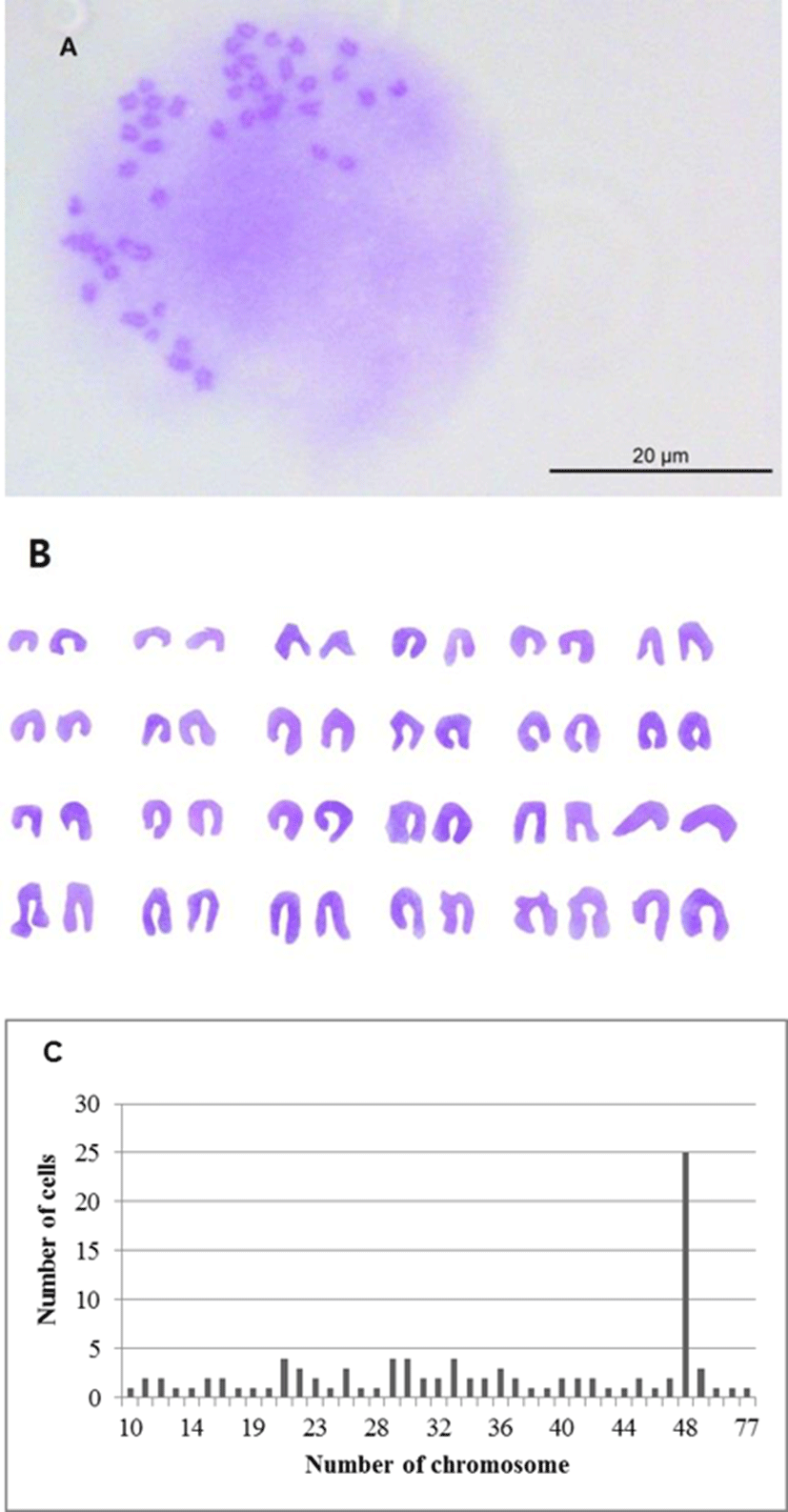
Chromosome morphology of OFEC-17FEN is presented in Fig. 3. The chromosome numbers of the OFEC-17FEN cells were assessed at passage 21. The result of chromosome counts of 100 metaphase revealed that the chromosome number of OFEC-17FEN cells was widely distributed between 10 and 77, with a modal peak at 48 chromosomes, and 25% of all cells contained 48 chromosomes. Chromosome numbers above and below the diploid number (Fig. 3), can be explained either by an incorrect segregation that can occur at low rate under cell culture conditions or more frequently by technical artifacts inherent to the obtention of chromosome spreads (Bejar et al., 2002).
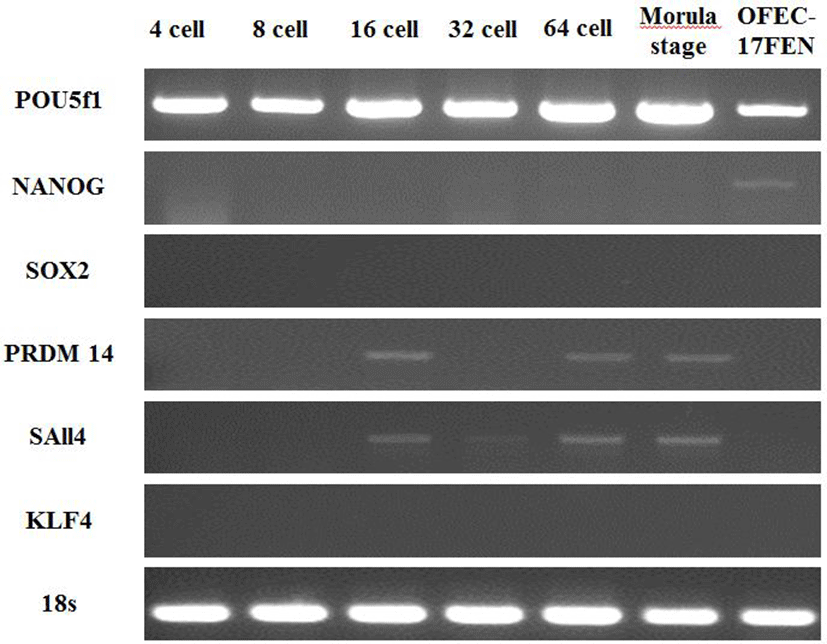
Expression of pluripotency genes was evaluated in OFEC-17FEN cells and in 4-, 8-, 16-, 32- and 64-cell-stage and morula-stage embryos. The expected 315 bp fragment of POU5f1, 300 bp fragment of NANOG, 503 bp fragment of SOX2, 300 bp fragment of SAll4, 305 bp fragment of PRDM14 gene, 300 bp fragment of KLF4, and 217 bp fragment of 18S were analyzed by 1.5% agarose gel electrophoresis. POU5f1 expression was high in the embryos and moderate in OFEC-17FEN cells. NANOG expression was low in OFEC-17FEN cells and not detected in the embryos. SOX2 was not expressed in the embryos or OFEC- 17FEN cells. PRDM14 expression was low in the 16- and 64-cell-stage embryos and absent in the other stage embryos and OFEC-17FEN cells. KLF4 was not expressed in OFEC-17FEN cells or the embryos. SAll4 expression was low in the 16-, 32- and 64-cell-stage, as well as morula stage, embryos but was not detected in OFEC-17FEN cells (Fig. 4).
To determine the transfection efficiency and gene expression of the OFEC-17FEN cells, cells were transfected with plasmid pEGFP-c1 by using JetPEI reagent. OFEC-17FEN cells transfected with pEGFP-c1 exhibited a strong green fluorescent signal at 48 h after transfection. The transfection efficiency of OFEC-17FEN cells was approximately 10%-15% (Fig. 5). Thus, OFEC-17FEN cells are useful for exogenous gene expression which is important for both basic research and biotechnological application.
DISCUSSION
In this study, olive flounder (Paralichthys olivaceus) embryonic cell lines capable of prolonged in vitro culture were developed and characterized in terms of their proliferation, karyotype exogenous gene expression and transfection ability.
OFEC-17FEN cells had been continuously cultured. Morphological appearance of OFEC-17FEN cell line was primarily epithelial-like cell. In this study, Fish serum is useful for primary cell culture and promoted in vitro growth of olive flounder embryonic cells, as reported in other fish species, e.g. sea perch (Lateolabrax japonicus), red sea bream (Chrysophrys major) and turbot (Scophthalmus maximus) (Chen et al., 2003a; Chen et al., 2003b; Chen et al., 2004; Chen et al., 2005). Furthermore, there is reported that Egg Extract (EE) has mitogenic activity in fish embryos (Bejar et al., 2002) and early developmental EE reportedly promotes cell migration and growth and thus can accelerate establishment of novel cell lines (Akiduki, 2010). Thus, use FS mixed with EE in media is essential for the primary cell culture of flounder embryo. This result is interesting and meaningful finding.
Karyotype analysis of developed cell lines is important (Chen et al., 2004), particularly to assess the ability to form functional germ-line chimeras (Chen et al., 2003b). Colchicine is used in karyotype analysis because of its ability to inhibit metaphase and condense chromosomes (Foresti et al., 1993). OFEC-17FEN cells had a 2n=48 diploid chromosome, identical to olive flounder (2n=48) (Liu et al., 1999). Approximately 25% of OFEC-17FEN cells had 48 chromosomes, which is lower than that in other fish species (Sun et al., 1995; Hong et al., 1996; Chen et al., 2003a; Peng et al., 2016), and may be due to metamorphosis and senescence during culture, however, these phenomena were not analyzed in this study. So further investigations involving metamorphosis and senescence about OFEC-17FEN cells will be require.
The pluripotency of the cell lines was assessed by RT-PCR. POU5f1, a POU domain transcription factor better known as OCT4, is critical for maintenance of the pluripotency and self-renewal of inner-cell-mass cells and for germ-line development in mice (Lachnit et al., 2008). OCT4, also known as OCT3, is involved in the self-renewal of embryonic stem cells (Rajpert et al., 2004). SOX2 is critical for maintaining the pluripotency of embryonic and neural stem cells (Gao et al., 2014). NANOG, in conjunction with OCT4 and SOX, is involved in maintaining the pluripotency and self-renewal of embryonic stem cells (Gao et al., 2013). PRDM14 has an N-terminal PR domain and six Krüppel-type zinc finger motifs and directly regulates POU5f1 through its proximal enhancer (Fan et al., 2015). KLF4 is highly expressed in post-mitotic gut and skin cells (Yu et al., 2011a). KLF4 is expressed in embryonic stem cells, and forced expression of OCT4, SOX2 and KLF4 induces reprogramming of fibroblasts into induced pluripotent stem cells (Wernig et al., 2007; Yu et al., 2011b). The SALL4 zinc finger transcription factor was first cloned based on its sequence homology to its orthologue in Drosophila spalt (Yang et al., 2007). In this study, SALL4 is expressed in two cell-stage embryos, similar to OCT4, whereas expression of SOX2 and NANOG is first shown at the blastocyst stage (Yang et al., 2010). Moreover, the expression of POU5f1 was high and that of NANOG was low, whereas SOX2, PRDM14, SALL4 and KLF4 were not expressed, in OFEC-17FEN cells. Consequently, OFEC-17FEN cells are not pluripotent. However, POU5f1 and NANOG expression suggested that OFEC-17FEN cells may have self-renewal ability. Accordingly, further investigations are needed to provide more in-depth analysis of relationship between OFEC-17FEN cells function and self-renewal ability.
The OFEC-17FEN cell density was highest on day 7 and decreased thereafter, likely due to nutrient depletion (Servili et al., 2009); therefore, OFEC-17FEN cells should be subcultured after 7 days. OFEN-17FEN cells proliferated more slowly than the other cell lines but displayed a similar growth curve.
In this study, OFEC-17FEN cells exhibited a transfection rate of 10%-15%. Primary cells are typically difficult to transfect, but this can generally be overcome by modifying the transfection protocol (Sassen et al., 2017). The successful transfection of pEGFP-c1 plasmid DNA suggests that OFEC-17FEN cells could facilitate exogenous gene manipulation in vitro (Huang et al., 2011).
In conclusion, the OFEC-17FEN cell line will be useful for basic research and biotechnological application.
