INTRODUCTION
Coiled-coil domain containing 110 (CCDC110, KM-HN-1) was initially discovered as a member of the family of human cancer/testis antigens (CTAs), in an attempt to find cancer-specific target proteins for the establishment of cancer treatment modalities and diagnostic methods (Monji et al., 2004). By using RT-PCR analysis of diverse tumor samples and cancer cell lines, the mRNA level of CCDC110 was found to be increased in several cancer types, including tongue cancer, stomach cancer, melanoma, and hepatocellular carcinoma (Monji et al., 2004; Condomines et al., 2007). CTAs are a group of proteins that were reported to exhibit high expression of proteins in specific types of cancers or testicular germ line cells (Chen et al., 1997; Scanlan et al., 2002; Vaughan et al., 2004). Due to their limited protein expression in somatic tissues and escape from immunological surveillance of testicular tissues, they have been considered as a promising target for the establishment of anticancer therapeutics and cancer diagnosis (Chen et al., 1997; Scanlan et al., 2002; Vaughan et al., 2004). To date, more than 270 genes were listed as encoding CTAs in the CTDatabase (http://www.cta.lncc.br) (Chen et al., 1997). CCDC110 contains a C-terminal coiled-coil domain (CCD) in which the C-terminal region of the SMC (structure maintenance of chromatin) structural motif resides. This motif is derived from the SMC protein, which plays an important role in chromosome segregation during anaphase (Cobbe & Heck, 2000). CCDC110 also includes a bipartite nuclear localization signal (NLS) flanked by a leucine zipper (ZIP) region, in the inner 1/3 region (Boulikas, 1993). The ZIP motif of CCDC110 is consist of 5 leucine-rich repeats (L1, L2, L3, L4, and L5) with intervening six-amino acid residues, and followed by basic amino acids residues like other ZIP motif-containing proteins (Park et al., 2007). The ZIP motif provides a protein-protein interaction domain of a variety of DNA binding proteins (Landschulz et al., 1988).
The role of CCDC110 in cell proliferation and carcinogenesis remains unclear. The NCBI’s deposited cDNA microarray data sets (http://www.ncbi.nlm.nih.gov/sites/GDSbrowser) about CCDC110 show evidence that CCDC110 may be related to cell proliferation. The mRNA expression of CCDC110 increases during the period of spermatogenesis, in pachytene spermatocytes and round spermatids (NCBI GEO GDS2390) (Namekawa et al., 2006), in the regenerating liver of mice (NCBI GEO GDS2577) (Otu et al., 2007), and under the overexpression of EGR gene in human umbilical vein endothelial cells (HUVEC) (NCBI GEO GDS2009) (Lucerna et al., 2006). Previously using adenovirus adopted overexpression system, we have identified that ectopically overexpressed of CCDC110 distinctly localized to centrosome and the C-terminal region of the protein seems to be the principal determinant of positioning this protein to the centrosome (Park et al., 2007). The deletion mutant lacking C-terminal CCD displays distinct nuclear localization pattern (Park et al., 2007). Here, we raised monoclonal antibodies against CCDC110 protein and tested it for feasibility to use in immunohistochemical staining (IHC). Ectopic overexpression of CCDC110 using the Tetracycline (Tet) inducible system led to delayed G2/M entry in U2-OS cells.
MATERIALS AND METHODS
Mouse hybridoma clone cells were maintained in RPMI 1640 medium (Hyclone Laboratories, Logan, UT, USA) containing the hypoxanthine-thymine (HT) supplement (Sigma-Aldrich, St Louis, MO, USA) and 10% fetal bovine serum (FBS; Hyclone Laboratories). U2-OS (ATCC, HTB-96), Tet-On U2-OS (Clontech Laboratories, Mountain View, CA, USA), and HEK- 293T(ATCC, CRL-3216) were maintained in Dulbecco’s modified Eagle medium (DMEM, Hyclone Laboratories), supplemented with 10% FBS and 100 U/mL of penicillin G plus 100 μg/ mL of streptomycin (HyClone Laboratories) at 37°C in a 5% CO2 humidified atmosphere.
The coding region of CCDC110 gene corresponding to N-terminal amino acid residue 1-417 of CCDC110 protein (CCDC110N) was amplified by PCR and sub-cloned into the pGEX4T-1 vector (GE Healthcare, Chicago, IL, USA), an expression vector for glutathione S-transferase (GST)-fusion protein. Bacterial expression of the GST-tagged CCDC110 protein was induced by the addition of 0.4 μM isopropyl-1-thio-β-D-galactopyranoside (IPTG) and subsequent cultivation for 18 h at 23°C. The purification of GST-CCDC110N protein was carried out using glutathione (GSH)-agarose affinity chromatography purification. The purified proteins were used to immunization and enzyme-linked immunosorbent assay (ELISA).
The production of monoclonal antibody was carried out from 2005 to 2007. 70 μg of the purified recombinant GST-CCDC110N protein was emulsified with Complete Freund’s Adjuvant (Sigma-Aldrich) and subsequently injected to 6-week-old female Balb/c mice. The boosting immunization was carried out two times for 2-week interval, each immunization with the same amount of purified proteins emulsified with incomplete Freund’s adjuvant (Sigma-Aldrich). Generation of GST-CCDC110N-specific antibodies in the serum of immunized mice was tested by both ELISA and immunoblot prior to hybridoma fusion. The hybridoma fusion was performed by standard techniques (Harlow & Lane, 1988). The immune cells harvested from spleens were fused with SP2/0-Ag14 murine myeloma cells in the presence of polyethylene glycol (PEG) 1500 (Roche, Mannheim, Germany). The obtained hybridoma cell mixtures were transferred into 96- well cell culture plates with the method of limiting dilution. The culture soups containing mAbs from the hybridoma cultures were screened by ELISA followed by immunoblotting. Ascites fluid was also produced by peritoneal injection of hybridoma cells into a pristane primed Balb/c mice.
SDS-PAGE and Immunoblotting was performed as described previously (Lee, 2012).
For immunoblotting analysis the following commercial primary antibodies were used in this study: anti-Cyclin D1 (#2922) and anti-Cyclin D3 (# 2936) were purchased from Cell Signaling Technology (Danvers, MA, USA); anti-Cyclin B1 (H-433), anti-p53 (SC-126), and anti-ERK1 (SC-94) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-p21 (ab7960) was purchased from Abcam (Cambridge, MA, USA). The primary antibodies were visualized with goat anti-rabbit (SC-2004, Santa Cruz Biotechnology, Santa Cruz) or goat anti- mouse (SC-2005, Santa Cruz Biotechnology) antibodies conjugated with horseradish peroxidase and an enhanced chemiluminescence (ECL) detection system.
The N-terminally enhanced green fluorescence protein (EGFP)-fused full-length open reading frame (ORF) of CCDC110 was PCR amplified and subcloned into pHR CMV*-1 puro SV40 vector and used for generation of lentivirus according to previous report (Soung et al., 2006). The pHR CMV*-1 puro SV40 EGFP-CCDC110 was transfected with pHRCMV-VSVG and pHRCMV-d8.2 dpr into HEK-293T cells and the culture soup containing lentivirus capable of expressing EGFP-CCDC110 and puromycin N-acetyltransferase (PAC) genes. The obtained lentivirus was introduced to Tet-On U2-OS cells and puromycin selection (2 μg/mL) was carried out. With the method of limiting dilution and immunoblotting, the Tet-inducible EGFP-CCDC110 U2-OS cell lines were cloned and used for further experiment.
U2-OS cells were grown on Poly D-lysine (Sigma-Aldrich)-coated glass coverslips. After wash-out with phosphate-buffered saline (PBS), cells were fixed with 3.7% formaldehyde solution dissolved in PBS, and then permeabilized with ice cold methanol for 2 min. After blocking with PBS containing 5% BSA solution, the cells were incubated for 2 h with each primary antibody. After wash-out the primary Abs, the cells were incubated with Alexa 532-conjugated anti- mouse IgG (Invitrogen, Carlsbad, CA, USA) was carried out for 2 h at room temperature. For the visualization of nucleus, Hoechst 33452 (Sigma-Aldrich) was added during the period of 1st washing after secondary antibody application. The stained cells were mounted on glass slides with semi-solidifying mounting solution (Polysciences, Warrington, PA, USA). Confocal fluorescence images were obtained by Carl Zeiss LSM 700 Meta microscope system (Carl Zeiss, Thornwood, NY, USA).
The IHC experiment using human tissue samples was approved from Dankook University Hospital IRB in 2006. The paraffin embedded tissue blocks of human testis previously obtained from a patient in his 50s who was hospitalized after a car accident were cut into 10-μm sections and placed on frosted glass microscope slides. After removal of paraffin with xylene, the tissue sections were dehydrated in a graded alcohol series. For the procedure of antigen retrieval, the tissue sections were heated in a pressured chamber containing 10 mM sodium citrate buffer (pH 6.1) for 3 min. After blocking of endogenous peroxide activity using 0.03% hydrogen peroxide, the sections were incubated for 2 h with a primary antibody (1:1 to 1:2 diluted culture soup for mAbs, 1:1,000 for polyclonal antibody) against CCDC110 at room temperature. The samples were washed and then incubated with HRP-conjugated anti-mouse IgG (Dako EnVision+system-HRP [DAB], Dako, Carpinteria, CA, USA) for 20 min at room temperature. After washing, the chromogen was developed for 2 min. The tissue sections were then counterstained with weak hematoxylin. The images of IHC was obtained using Olympus BX51 upright microscope (Olympus, Tokyo, Japan) equipped with digital camera.
RESULTS
With ELISA screening, nine hybridoma clones reactive with CCDC110 protein were obtained. The isotypes of each CCDC110 mAbs were tested and determined (Table 1). The reactivity of all mAbs against both endogenous and overexpressed CCDC110 proteins was tested (Fig. 1A). As shown in Fig. 1A, each clone of the mAbs readily detects overexpressed recombinant proteins. However, the detection of endogenous CCDC110 protein around 105 kDa seems to be elusive.
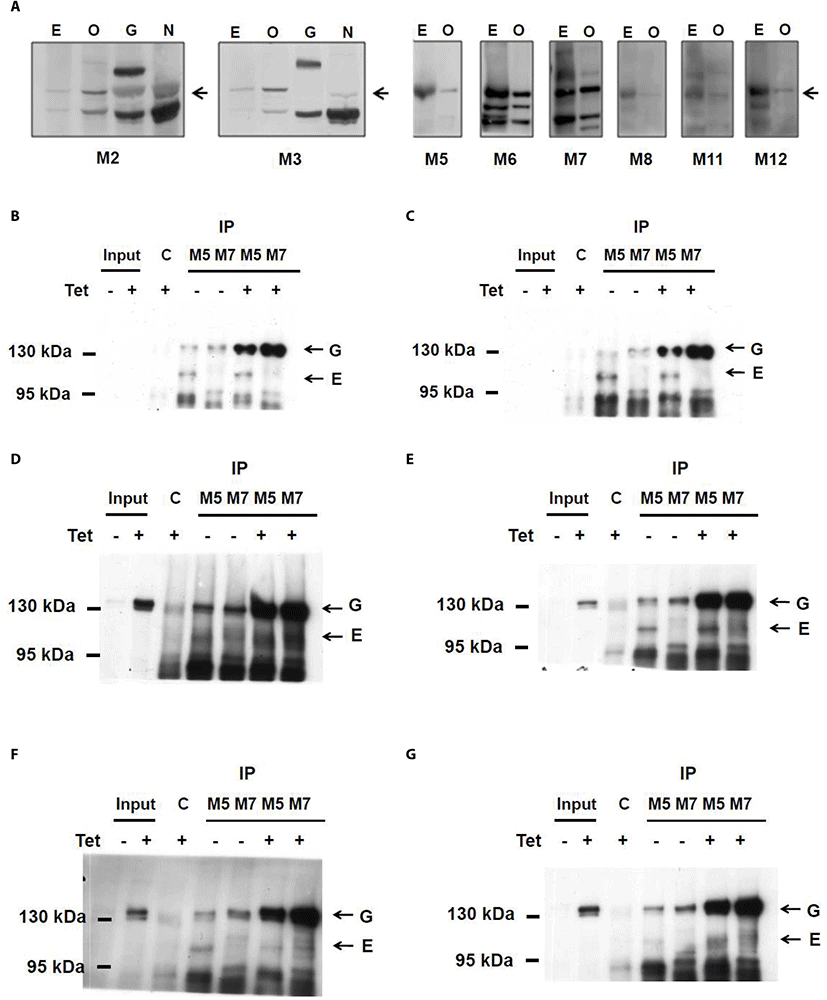
The results of isotyping, specificity and sensitivity of Immunoblotting, and the efficacy of immunoprecipitation was presented. E, untransfected cell lysate; OE, detection of ectopically overexpressed protein; IP immunoprecipitation. The immunoprecipitation efficacy of M5 and M7 mAbs were assessed.
The relative immunoblotting reactivity is divided into four levels: ±, +, ++, +++.
To test whether mAbs can immunoprecipitate CCDC110 protein, we used Tet-inducible U2-OS cells which can produce EGFP-CCDC110 protein upon treatment with tetracycline or their related delivatives. The proteins obtained from the lysates of both doxycycline-treated and untreated cells were immunoprecipitated with hybridoma mAbs and blotted with obtained mAbs (Fig. 1B–G). M5 Ab reacted with both endogenous and overexpressed CCDC110. However, M7 clone can specifically immunoprecipitate the overexpressed CCDC110 but not the endogenous CCDC10. The immunoprecipitated endogenous proteins can be detected by M2, M3, M5, M8, and M11 Abs which can also detect overexpressed CCDC110. Although the immunoprecipitated endogenous proteins could be detected in the IP sample, the corresponding signals of the protein in cell lysate (input) were seldom detected. The characteristics of each clone are summarized in Table 1.
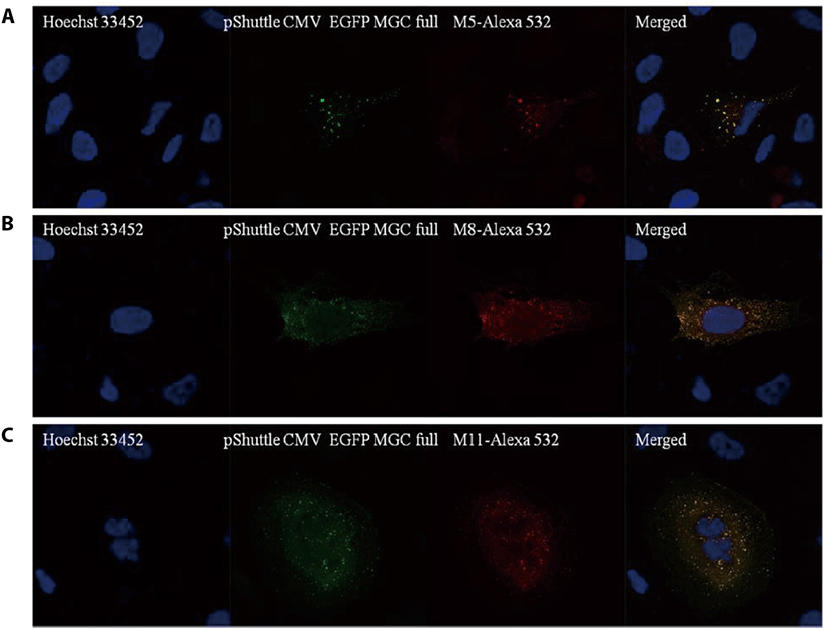
To test whether the CCDC110 proteins can be detected by mAbs, U2-OS cells were transfected with pShuttle CMV EGFP MGC full, which can express CCDC110 fused with EGFP, and immunofluorescence staining was conducted. As shown in Fig. 2, hybridoma clones M5, M8, and M11 can detect ectopically overexpressed EGFP-CCDC110 protein. The green fluorescence signal of EGFP colocalized with the red fluorescence signal of each mAbs stained with Alexa 532 conjugated anti-mouse IgG. These mAbs were further tested in IHC of tissue sections.
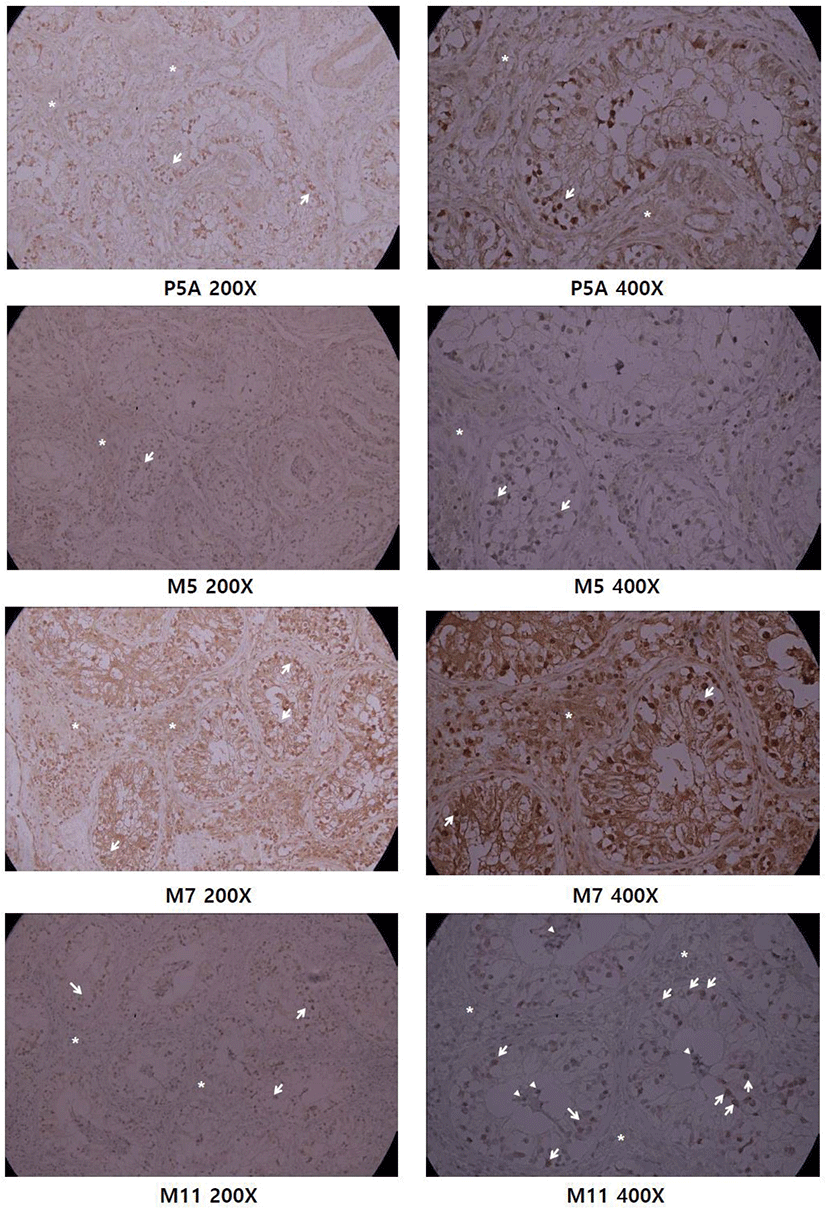
Previously we raised polyclonal antisera and purified it with GST-CCDC110 affinity purification (Park et al., 2007). We tested anti-CCDC110 mAbs for IHC of paraffin-embedded testis block and compared with affinity-purified polyclonal Ab (Fig. 3). Polyclonal Ab showed a strong signal in the seminiferous tubule area, whereas no signal was detected in the stromal region. The IHC using M7 antibody showed high background signal in the extra-tubule area. In 200× magnification, the M5 and M11 mAbs share a similar IHC staining pattern: prominent in the tubular basal area and weak in the stromal area, which is similar to affinity-purified polyclonal Ab (P5A). The IHC of M11 mAb shows a more significant difference between parenchymal spermatogenic cells and stromal cells, observed both in 200× and 400× magnification. The 400× magnification of M11 IHC reveals both nuclear and cytoplasmic staining in spermatogenic cells in the basal region of tubule abundant of spermatogonia. As the cells in the seminiferous tubule area have high replication potential due to spermatogenesis compared with those in the stromal region in which connective tissues and fibroblasts exist, the Ab seems to detect highly replicative spermatogenic cells.
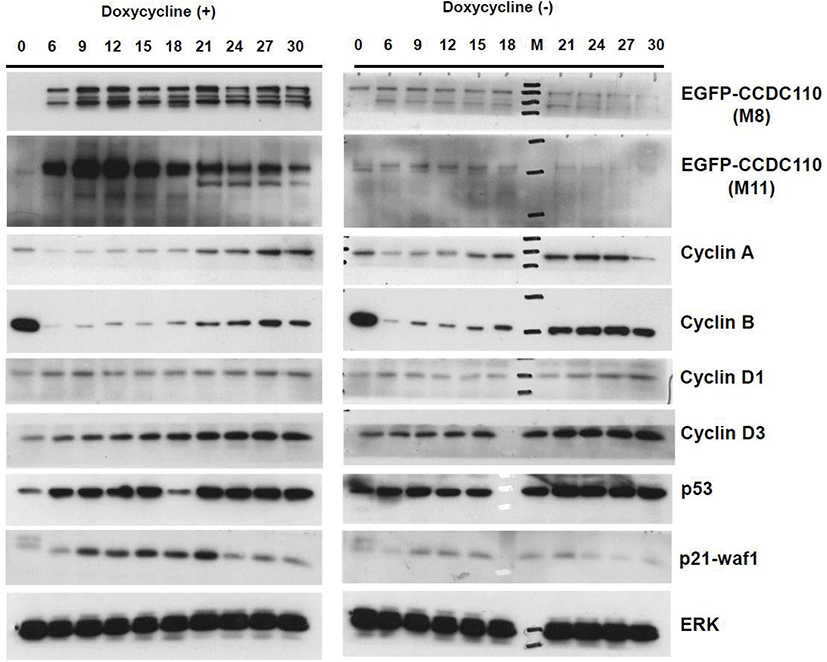
The Tet-On U2-OS cells carrying inducible EGFP-CCDC110 protein were synchronized with mitotic phase arrest by nocodazole treatment followed by mitotic exit and entry into G1 phase by shake-off plating. The levels of cell cycle markers were evaluated (Fig. 4). As expected, the nocodazole arrested cells showed highest levels of cyclin B. The signals of cyclin B reappear at 9 h and show peaks at 21–27 h after 1st mitotic exit in doxycycline untreated control cells. However, the increase of cyclin B in the Tet-induced EGFP-CCDC110 expressing cells was delayed and suppressed. The increase of cyclin B in EGFP-CCDC110-overexpressed cells coincided with the gradual decrease of EGFP-CCDC110. The expression of cyclin A also shows a delayed increase, similar to cyclin B. The level of induced EGFP-CCDC110 is disappeared in nocodazole treated M phase-arrested cell and increases at 6 h, peaked at 12 h and decreases gradually. The level of p21 was increased in EGFP-CCDC110-induced cells compared to uninduced cells. The immunoblot signals of endogenous CCDC110 was too weak to detect under these conditions.
DISCUSSION
CCDC110 is one of the members of CTAs and its expression is minimal in normal tissues but is increased in testis tissues and several types of cancers. Testicular tissues show the characteristic high activity of cell proliferation due to spermatogenesis in the seminiferous area and the proteins specific to testicular tissues are generally not exposed to self-immune defense system (Chen et al., 1997). CTAs are known to be a the valuable druggable candidate for immunotherapy or cancers (Chen et al., 1997; Scanlan et al., 2002; Scanlan et al., 2004). In addition, CCDC110 contains C-terminal CCD and ZIP repeats at amino acid residues 448–483. It also contains NLS motif at 338–355 and SMC motif at 431–790 (Park et al., 2007). The ZIP motif is important for protein oligomerization and CCDC110 has five repeats of ZIP exceeding the minimal requirement number of repeats. Several microarray results showed that the CCDC110 protein increases in cells or conditions where cell proliferation is active. However, little is known about cellular function except for characteristic overexpression of mRNA levels in various cancer tissues such as tongue cancer, melanoma, and liver cancer. A recent study showed that CCDC110 auto-antibodies are detected in the cohort of gastric cancer patients (Hoshino et al., 2017).
In this work, we developed an efficient CCDC110 mAb because the production of mAb against CTAs is useful for its clinical application as cancer markers in pathology, serological diagnosis and prediction of cancer prognosis. All the obtained mAbs showed stronger reactivity in immunofluorescence staining of exogenously overexpressed proteins. The immunohistochemical analysis of mAb M11 revealed similar or better outcome with affinity-purified polyclonal antibody. However, we did not compare the efficacy of our developed antibodies with the commercially available antibodies. The M11 mAb was strongly stained in cells in the basal layer of tubules rich in spermatogenic cells and negatively stained in stromal connective tissue regions. The M11 stained signal exists both in the cytoplasm and nucleus, showing that the M11 is suitable for application of IHC targeting CCDC110 in various cancer tissues. Thus, we believe that this mAb will be useful for characterizing the expression levels of CCDC110 and its functional role in various cancer tissues.
Notably, we found that the levels of overexpressed CCDC110 inversely correlated with the levels of cyclin B in TRE harboring Tet-On U2-OS cells, suggesting that this protein is under the control of certain protein degradation system activated in the G2/M phase. However, we cannot rule out the possibility that this result is caused by potential artifacts associated with lentiviral infection in the engineered cell line used in our study. Nevertheless, our results suggest that CCDC110 can be regulated during cell cycle progression and its persistent expression can impede cell cycle progression. However, it is also plausible that the degradation of overexpressed CCDC110 is mediated by dysregulated protein process under our ectopic expression condition. Further study is needed to elucidate a more detailed molecular mechanism associated with cell cycle-controlled degradation of CCDC110.
