INTRODUCTION
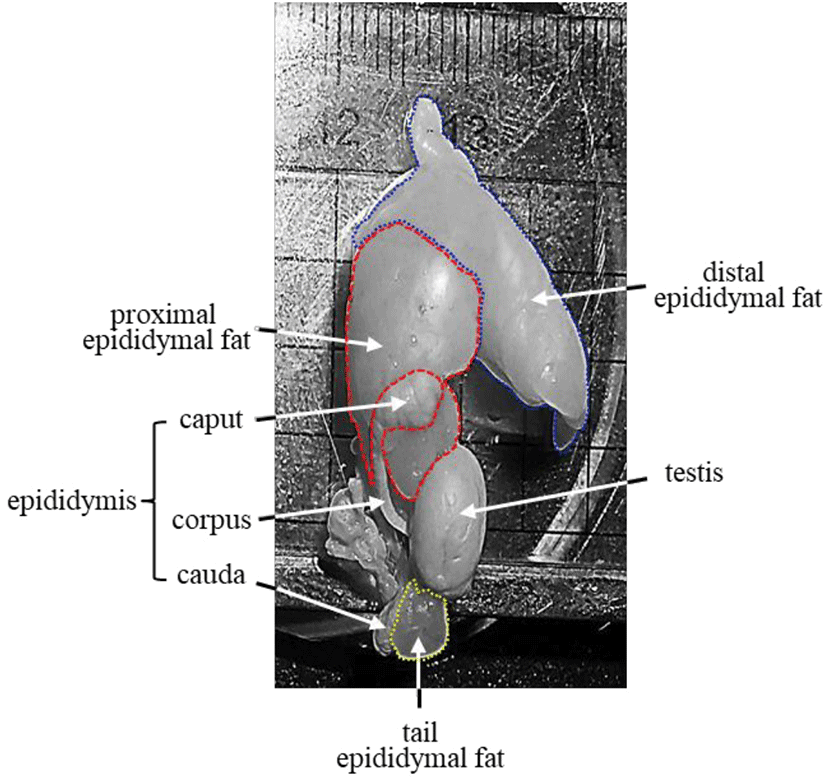
It is well defined that the epididymal fat is located near the head of the epididymis (Pond, 1999; Berry et al., 2013) (Fig. 1). As a part of gonadal adipose tissue, the epididymal fat is divided into two parts, distal and proximal epididymal fat, based on histochemical characteristics (Tirard et al., 2007). For instance, the proximal epididymal fat has higher aldo-keto reductase 1B7, cyclooxygenase 2, and GATA-3 transcript levels than the distal epididymal fat (Tirard et al., 2007). In addition, our recent researches have shown different expression patterns of some adipocyte-associated molecules during postnatal period (Lee, 2019; Lee & Kim, 2019). Expression of leptin (Lep) in the distal epididymal fat is continuously increased as aged (Lee & Kim, 2019), while the highest level of Lep in the proximal epididymal fat is detected at 8 months of age, followed by a significant drop at 12 months of age (Lee, 2019). Similar findings are found in the expression patterns of fatty acid synthase (Fasn) and lipoprotein lipase (Lpl), resistin (Retn), adiponectin (Adipoq), and fatty acid binding protein 4 (Fabp4) (Lee, 2019; Lee & Kim, 2019). Differential expression patterns of adipocyte-associated genes in two epididymal fat regions suggest that these two regions of epididymal fat would have different developmental pathways and thereby biological functions.
The epididymal fat is derived from non-adipogenic multipotent mesenchymal progenitor cells at the very early postnatal age (Han et al., 2011). The differentiation and development into the pre- and mature epididymal adipocyte of the progenitor cells are dependent upon the cell-matrix and cell-cell contact and appear to begin as early as 4 days of postnatal age in the mouse (Han et al., 2011). The mature adipocyte-like cells are first observed around 14 days of postnatal age, and the distal epididymal fat likely contributes to proper development of the epididymal fat at the adult (Han et al., 2011). The development of epididymal fat during postnatal period is associated with enlargement of size and increase of adipocyte number (Cleary et al., 1977). The growth of epididymal fat at the early stage of postnatal development is mostly related with proliferation of adipocyte, while the size expansion of each adipocyte particularly influences on the development of epididymal fat at late postnatal stage (Cleary et al., 1977; Gruen et al., 1980). Even though the detailed molecular mechanism of epididymal fat growth during postnatal period has not been addressed, it is believed that the general pathway involving in sequential actions of various adipogenic genes required for proliferation and maturation of adipocyte would be applicable to postnatal development of the epididymal fat (Hausman et al., 2001).
Other than the ordinary epididymal fat placed between the testis and the epididymis, an additional fat deposit, named the tail epididymal fat, exists near the caudal epididymis (Fig. 1). The tail epididymal fat is much smaller than the ordinary epididymal fat and appears to begin around 10 days of postnatal age (personal observation). Like the ordinary epididymal fat, the tail epididymal fat continuously grows in size as aged (personal observation). However, it is hard to find detailed description of the tail epididymal fat, and no careful examination of the tail epididymal fat has not been conducted yet. In spite of a lack of scientific information about the tail epididymal fat, the being at anatomically different place from the ordinary epididymal fat has been held an attention for the biological examination.
Our previous researches have shown the existence and different expression patterns of some adipocyte-associated molecules in the distal and proximal epididymal fat in mouse and whole epididymal fat in rat at various postnatal ages (Lee & Kim, 2018; Lee, 2019; Lee & Kim, 2019). Thus, the present research was focused to determine whether expression of adipocyte-associated molecules found in the ordinary epididymal fat could be detected in the tail epididymal fat using quantitative real-time polymerase chain reaction (PCR). In addition, expression patterns of these molecules in the tail epididymal fat during postnatal period was compared with those in the ordinary epididymal fat.
MATERIALS AND METHODS
A total of 24 male C57BL/6N mice at 1 month of postnatal age were purchased from Samtako (Osan, Korea), and randomly allocated into 4 different age groups, 2, 5, 8, and 12 months of age, at the arrival. Each age group had six mice, and experimental animals were allowed for food and water ad libitum under controlled environment (14-h/10-h light/dark cycle) for a whole experimental period. All procedures were carried out in accordance with the guidelines for the care and use of laboratory animals of National Research Council in Korea.
When the animal reached a proper postnatal age, the animal was euthanized by CO2 stunning in a closed plastic chamber. The male reproductive tract, including testis, epididymis, and epididymal fat pad, was pulled out throughout a lower abdominal incision and was placed in cold PBS. The epididymis was separated from the testis, and the tail epididymal fat near the caudal epididymis was carefully isolated from the rest of epididymal part and vas deferens. The collected tail epididymal fat was washed once in fresh cold PBS and rapidly placed in liquid nitrogen and then −80°C freezer. Because the tail epididymal fat collected from an experimental animal is not sufficient to obtain total RNA aliquot for quantitative real-time PCR analysis, all tail epididymal fat tissues in an experimental age group were pooled to acquire adequate amount of total RNA extract.
A pool of the tail epididymal fat was homogenized in 1 mL of TRI REAGENT solution (Molecular Research Center, Cincinnati, OH, USA). The total RNA pellet was obtained from sequential addition of chloroform and isopropanol into tissue homogenate and resuspended in DEPC-treated dH2O. The quantity and quality of total RNA extract were determined by a spectrophotometry (NanoDrop Lite, Thermo Scientific, Wilmington, DE, USA) and 1.2% agarose gel electrophoresis, respectively.
To generate the first strand of complementary DNA (cDNA) for quantitative real-time PCR analysis, 1 μg of total RNA was mixed with pre-made reverse transcription (RT) mixture (iScrip™ Reverse transcription Supermix for RT-qPCR, Bio-Rad Laboratories, Hercules, CA, USA) and nuclease free-dH2O to make the final volume of 20 μL. The RT reaction was carried out in 25°C for 5 min, 46°C for 20 min, and 95°C for 1 min.
The constructed cDNA was directly utilized for quantitative real-time PCR analysis. The same PCR primers employed from previous researches for mouse distal and proximal epididymal fat tissue (Lee, 2019; Lee & Kim, 2019) were used for the current study. The detailed information for oligonucleotide primers is listed in Table 1. The PCR mixture was prepared with 1 μL of cDNA, 10 pmol of each primer, 7 μL of iQ™ SYBR® Green Supermix (Bio-Rad Laboratories), and nuclease free-dH2O to adjust the final reaction volume. The PCR condition is as follows; a pre-denaturation at 95°C for 5 min, cycles of a denaturation at 95°C for 30 sec, an annealing at Tm for 30 sec, and an extension at 72°C for 30 sec, and an additional extension at 72°C for 10 min. The presumed size of PCR product was checked with 1.2% agarose gel electrophoresis.
PCR, polymerase chain reaction; Pparg, peroxisome proliferator-activated receptor, gamma; Fabp4, fatty acid binding protein 4; Dlk1, delta like non-canonical Notch ligand 1; Fasn, fatty acid synthase; Lpl, lipoprotein lipase; Lep, leptin; Adipoq, adiponectin; Retn, resistin; Rn18s, 18S ribosomal RNA.
Independently quadruped RT reactions and PCRs were performed to obtain a mean and standard error for the expression level of each target molecule at a given postnatal age. The expression level of a target gene was normalized to that of 18S ribosomal RNA (Rn18s), an internal control of PCR analysis and was presented in the relative ratio against that of Rn18s in a graph.
The existence of statistical difference among the transcript levels of a target gene at different postnatal ages was first evaluated by one-way ANOVA. If significance present, a post-hoc analysis, Duncan’s test, was followed to determine statistical difference among age groups. If p-value was less than 0.05, it was judged as statistically significant on expression level of the target gene between those different ages and then denoted by different letters in the result graph.
RESULTS
Expression patterns of Pparg and Fabp4 are shown in Fig. 2. The level of Pparg transcript was significantly increased at 5 months of age, compared with its at 2 months of age (Fig. 2A). However, expression level of Pparg gene was not changed at 8 months of age and even at 12 months of age (Fig. 2A).
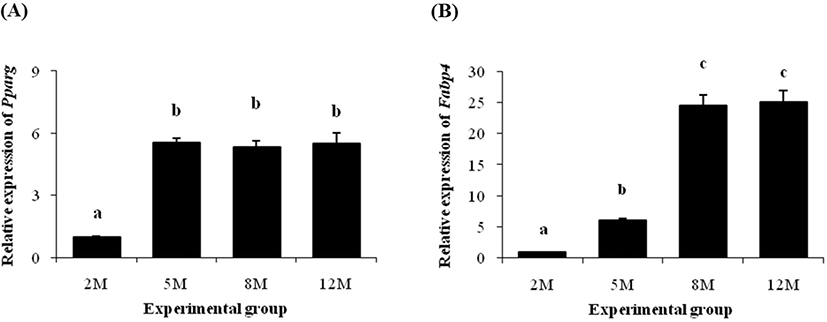
The transcript level of Fabp4 at 5 months of age was also significantly higher than its at 2 months of age (Fig. 2B). However, unlike Pparg, an additional huge increase of Fabp4 mRNA level was detected at 8 months of age (Fig. 2B). The expression level of Fabp4 at 12 months of age was not different with its at 8 months of age (Fig. 2B).
Expression level of Dlk1 gene at 5 months of age was significantly increased than its at 2 months of age (Fig. 3A). The transcript level of Dlk1 at 8 months of age was about 3-fold higher than its at 5 months of age (Fig. 3A). An additional over 2-times increase of Dlk1 transcript level was detected at 12 months of age (Fig. 3A).
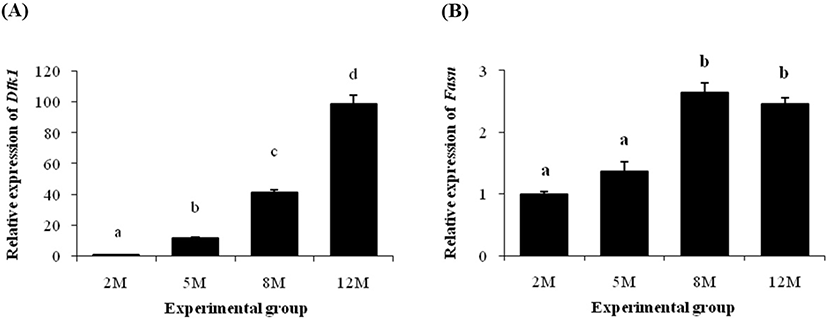
There was no significant change of Fasn transcript level at 5 months of age, compared with its at 2 months of age (Fig. 3B). However, the level of Fasn mRNA was significantly increased at 8 months of age, followed by a steady level at 12 months of age (Fig. 3B).
Expression of Lpl gene was significantly increased at 5 months of age, compared with the level of Lpl transcript at 2 months of age (Fig. 4A). A further increase of Lpl transcript level was observed at 8 months of age (Fig. 4A). However, there was no significant expression change of Lpl between 8 and 12 months of age (Fig. 4A).
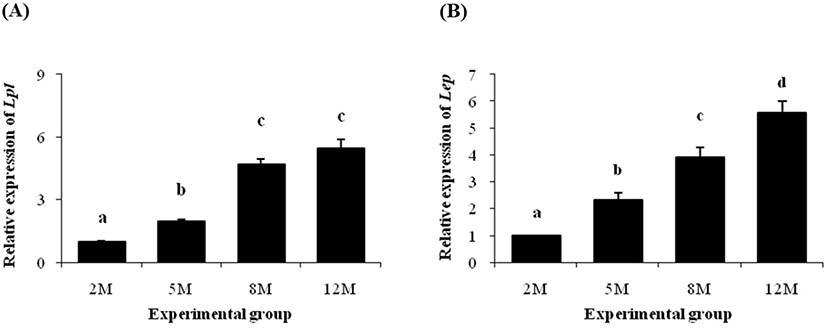
As shown in Fig. 4B, a significant increase of Lep transcript level at 5 months of age was detected, compared with its at 2 months of age. An additional increase of Lep mRNA level was found at 8 months of age, followed by a further rise of Lep transcript level at 12 months of age (Fig. 4B).
About 5-fold increase of Adipoq transcript level was detected at 5 months of age, compared with at 2 months of age (Fig. 5A). A further significant increase of Adipoq gene expression was observed at 8 months of age (Fig. 5A). But, the Adipoq transcript level at 12 months of age was not statistically different with its at 8 months of age (Fig. 5A).
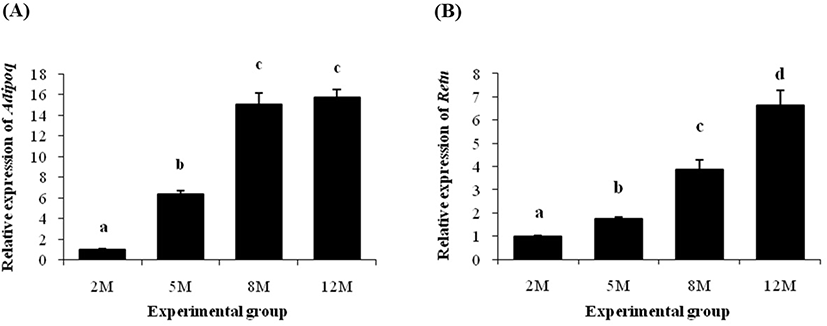
The transcript level of Retn at 5 months of age was significantly higher than its at 2 months of age (Fig. 5B). Then, an additional increase of Retn transcript level was detected at 8 months of age (Fig. 5B). The highest expression level of Retn gene among experimental age groups was found at 12 months of age (Fig. 5B).
DISCUSSION
The presence and nature of the tail epididymal fat have not been recognized, presumably due to small size and the location isolated from the ordinary epididymal fat. In the present research, the presence and expression patterns of adipocyte-associated molecules found in the ordinary epididymal fat at different postnatal ages have been investigated in the tail epididymal fat by quantitative real-time PCR analysis. The research data show that the transcripts of adipocyte-associated genes examined are indeed present in the tail epididymal fat. In addition, expression levels of these genes during postnatal period are mostly higher at the elderly age than those at the younger age.
The differentiation of preadipocyte into mature adipocyte and maintenance of differentiated status of adipocyte require a proper expression of Pparg (Tamori et al., 2002). Interestingly, the expression patterns of Pparg in the distal and proximal epididymal fat during postnatal period are slightly different (Lee, 2019; Lee & Kim, 2019). That is, the transcript level of Pparg in the distal epididymal fat is continuously increased as aged (Lee & Kim, 2019), while Pparg transcript level in the proximal epididymal fat is significant decreased at 5 months of age, followed by the highest level of Pparg at 8 months of age (Lee, 2019). In the current findings, the level of Pparg expression in the tail epididymal fat is significantly increased at 5 months of age and remained at constant level until 12 months of age. These results imply that the maturation of each epididymal fat segment by the action of Pparg is likely differentially regulated during postnatal period. From in vitro experiment with adipocytes derived from different epididymal fat parts, the modification of Pparg expression would provide information to assess a role of Pparg on the postnatal maturation of the epididymal fat.
The expression of Fabp4 in various adipocyte tissue has been revealed in previous researches (Kletzien et al., 1992). As a promoting factor for lipid storage in adipocyte, it is reasonable to consider that expression of Fabp4 would be increased as fat tissue becomes larger with aging. Indeed, the present showed that Fabp4 transcript levels at 8 and 12 months of age were over 20-folds higher than that at 2 months of age, indicating the occurrence of considerable accumulation of lipid droplet within the tail epididymal fat cells at the later postnatal age. Expression patterns of Fabp4 in the distal and proximal epididymal fat during postnatal period are not same with that in the tail epididymal fat. In the proximal epididymal fat, a significant increase of Fabp4 expression is observed at 8 months of age (Lee, 2019). However, Fabp4 transcript level in the distal epididymal fat is continuously increased as ages (Lee & Kim, 2019). The present study shows that expression pattern of Fabp4 in the tail epididymal fat until 8 months of postnatal age is quite similar with that in the distal epididymal fat, even though an additional significant increase of Fabp4 expression at 12 months of age is detected in the distal epididymal fat (Lee, 2019). From these observations, it is presumed that lipid accumulation in the distal, proximal, and tail epididymal fats actively occurs around 8 months of age.
The Dlk1 promotes an inhibition of adipogenesis and suppress the differentiation of preadipocyte into adipocyte, resulting in higher expression of Dlk1 in preadipocyte than mature adipocyte (Wang & Sul, 2009). In the distal and proximal epididymal fat, the extraordinary increases of Dlk1 transcript level are observed at 8 months of age (Lee, 2019; Lee & Kim, 2019). Even though the magnitude of surge in Dlk expression is slightly different, the Dlk transcript levels in the tail epididymal fat are also increased as aged. Together, these findings imply that Dlk plays a role on the maturation of the epididymal fat during postnatal period, other than an inhibitory effect on adipogenesis, because the differentiation of preadipocyte in the epididymal fat seems to be completed before 1 month of postnatal age (Han et al., 2011). An examination of functional role of Dlk in the differentiated adipocyte would be helpful to understand how Dlk influences on the maturation of the epididymal fat during postnatal period.
The LPL promotes lipolysis and FASN stimulates lipogenesis in adipocyte (Proença et al., 2014). Thus, the balanced actions of LPL and FASN are necessary to regulate the storage of fatty acid in adipocyte. Because the cell size and lipid storage of the epididymal adipocyte at the elderly age is larger than those at the younger age (Björntorp et al., 1979), it is considerable that expression of Lpl would be decreased and the level of Fasn transcript would be increased as aged. However, the present research has shown significantly high levels of both Lpl and Fasn expression at the elderly age. Similar expression patterns of Lpl and Fasn at the tail epididymal fat are observed at the distal and proximal epididymal fat during postnatal age (Lee, 2019; Lee & Kim, 2019). It is not possible to provide a clear explanation about the increase of Lpl expression in the epididymal fat with aging at this point. A possible suggestion would be that the increase of Lpl transcript level does not always reflect the increase of functional LPL protein level, due to different transcription and translation efficiencies of gene expression. An additional examination at protein level could deliver clear information to explain such expression pattern of Lpl in the epididymal fat during postnatal period. The crosstalk between adipocyte and metabolic tissues for regulation of systemic energy expenditure is usually controlled by adipocytokines secreted from the adipose tissue, such as leptin, adiponectin, and resistin (Haiming, 2014). The biological functions of these adipocytokines include the control of food intake and energy usage by leptin, reduced insulin resistance and thus increased insulin action by adiponectin, and an increase of insulin resistance by resistin (Haiming, 2014). The function of adiponectin in the male reproductive tract has not been clearly defined, in spite of the presence of adiponectin receptor type 1 and 2 in the testis and epididymis (Singh et al., 2018). A possible positive regulatory effect of resistin on steroidogenic activity in the testicular Leydig cell has been suggested (Roumaud & Martin, 2015). Even though the exact role of leptin on the male reproductive function has not been revealed, it is proposed that leptin would involve in suppression of testicular steroidogenesis (Zhang & Gong, 2018). These findings reflex the existence of a complex regulatory mechanism on the male reproduction by adipocytokines. Chu et al. (2010) have demonstrated that the presence of ordinary epididymal fat is essential for maintaining spermatogenesis, suggesting possible role of adipokines originated from the ordinary epididymal fat on the regulation of spermatogenesis in the testis. Thus, based on the findings of Lep, Adipoq, and Retn expression in the tail epididymal fat from the present study, it is reasonable to consider that the tail epididymal fat could have an effect on the testicular function.
The current findings have the significances on the examination of the presence and expression patterns of various adipocyte-associate genes in the mouse tail epididymal fat at different postnatal ages, presumably for the first time. Expression patterns of these genes distinguished from those in the ordinary epididymal fat imply the existence of distinct role of the tail epididymal fat. Even though the data from present research does not give a direct information of an importance of the tail epididymal fat, further examination of this tissue would provide an additional evidence of a functional role of epididymal fat on the male reproduction, such as regulation of testicular and/or epididymal function.
