INTRODUCTION
The olive flounder is a flatfish belonging to the Pleuronectiformes order and Paralichthyidae family. It is an economically important marine organism that accounts for 40% of the marine fish farming (Bai et al., 2010). However, culturing olive flounder is subjected to multiple challenges owing to the various diseases and poor breeding environments (Kim et al., 2003; Kim et al., 2009). One notable challenge has been the damage caused by viral hemorrhagic septicemia virus (VHSV) infection. Every year in the East and South Sea waters, VHSV largely infects olive flounder and other wild fishes as well. The infection occurs even during winter and spring, when the water temperatures are low. The most common visible changes observed in an infected fish are body color blackening, abdominal distension, and hernia. Gill fading during dissection and clear ascites in the abdomen are also characteristic of VHSV infected fishes (kim et al., 2009; Duesund et al., 2010).
Caspase is a type of cysteine proteolytic enzyme that plays an important role in various stages of cell death, such as apoptosis, necrosis, and inflammatory reactions (Thornberry & Lazebnik, 1998; Grutter, 2000). According to the primary functions, caspase can be classified into apoptotic caspases (caspase 2, 3, 6, 7, 8, 9, and 10) and inflammatory caspases (caspase 1, 4, 5, 11, 12, and 13) (Earnshaw et al., 1999; Galluzzi et al., 2016). Apoptotic caspases can be further classified as initiator caspases (caspase 2, 8, 9, and 10) and executioner caspases (caspase 3, 6, and 7). Initiator caspases are located upstream in the caspase signaling cascade and are responsible for receiving and transmitting the apoptotic signals from the mitochondria or death receptors to the effector caspases (Vincenz & Dixit, 1997; Pan et al., 1998). Executioner caspases further transmits the apoptosis-inducing signals from the initiator caspases to activate a variety of apoptosis-mediating enzymes, such as the deoxyribonuclease (DNase) and protease (Liu et al., 1997).
Caspase10 encoded by the caspase10 gene belongs to the cysteine-aspartic acid protease family and is responsible for maintaining cellular homeostasis by regulating the immune response and apoptosis (Yan et al., 2012; Kumari et al., 2019). Caspase10 acts on the effector procaspase and thereby cleaves it into its biologically active form that plays an important role in the Fas ligand mediated apoptosis (Stennicke et al., 1998; Ekchariyawat et al., 2012). Caspase10 is involved in the elimination of T cells in response to an autogenous antigen and thus results in an immune system disorder characterized by reduced sensitivity to Fas ligand mediated apoptosis (Shin et al., 2002).
Currently, studies have reported the presence of caspase family in Atlantic salmon (Salmo salar), channel catfish (Ictalurus punctatus), goldfish (Carassius auratus), medaka (Oryzias latipes), orange-spotted grouper (Epinephelus coioides), rainbow trout (Oncorhynchus mykiss), rohu (Labeo rohita), sea bass (Dicentrarchus labrax) and zebrafish (Daniorerio) (Naruse et al., 2000; Laing et al., 2001; Yabu et al., 2001; Long et al., 2004; Amali et al., 2006; Chakraborty et al., 2006; Takle et al., 2006; Reis et al., 2007a; Reis et al., 2007b; Arockiaraj et al., 2013; Allan et al., 2020; Samanta et al., 2020; Zhang et al., 2020). Caspase10 has been specifically reported in olive flounder, black rockfish (Sebastes schlegelii), and snakehead murrel (Channa striatus) (Kurobe et al., 2007; Elvitigala et al., 2015; Cho et al., 2016). There is limited information on the immune system regulation mediated by caspase10 in response to viral infection in the fishes. Therefore, in this study, we investigated the immune response of caspase10 to VHSV infection in olive flounder, one of the major causes of mass mortality.
MATERIAlS AND METHODS
The olive flounders used in the experiment were bred at 19±1°C in a 3-ton flow-through tank using a 15 h photoperiod and 9 h dark cycle, at the Genetics and Breeding Research Center, National Institute of Fisheries Science (NIFS). In order to analyze the expression of caspase10 during the different developmental stages of olive flounder, samples from 0 to 40 days after hatching (DAH) were placed in a TRI-Solution™ (TS200-001, Bio Science Technology, Daegu, and Korea) and stored at −80°C until ribonucleic acid (RNA) isolation. To analyze the tissue-specific expression of caspase10, various tissues were extracted from a healthy 8-month-old olive flounder (total length approximately 30 cm) and stored at −80°C until RNA isolation. To minimize the stress of olive flounder, anesthesia was performed using tricaine methanesulfonate (MS-222) (Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 150 ppm, before the experiment (Noh et al., 2017).
The VHSV artificial infection test was conducted using healthy 8-months-old olive flounder (total length approximately 30 cm). The fish were made to fast on the day before and on the day of artificial infection test after being placed in a 3-ton flow-through tank with water at a temperature of 19±1°C. A breeding condition of 15 h photoperiod and a 9 h dark cycle was maintained. The control group was inoculated with 100 μL of 1X phosphate buffered saline (PBS), while the experimental group was inoculated with 105 median tissue culture infectious dose (TCID50) of VHSV. The kidney, gill, skin, and spleen were sampled every 0, 1, 3, 6, 9, 12, and 18 h for the first five consecutive days after inoculation and storage at −80°C until RNA isolation. To minimize the stress of olive flounder, anesthesia was performed using MS-222 at a concentration of 150 ppm, before the experiment (Noh et al., 2017).
Total RNA was extracted using the same amount of sample by modifying the protocol of the TRI-SolutionTM. The extracted RNA was decontaminated for genomic DNA using DNase-I. Total RNA was spectrophotometrically (Eon™ Microplate Spectrophotometer, BioTek, Winooski, VT, USA) evaluated by the ratio of the absorbance at 260 and 280 nm (A260/280) and 260 and 230 nm (A260/230). Total RNA was then used for the synthesis of complementary DNA (cDNA) using oligo (dT)18 primer and Transcriptor First Strand cDNA Synthesis Kit (Roche, Mannheim, Germany). Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed using 100 ng/μL of cDNA under the following process conditions: initial denaturation at 95°C for 20 s, annealing at 58°C for 30 s and elongation at 60°C for 30 s. The final dissociation marked the completion of the qRT-PCR reaction. The primers used for the qRT-PCR analysis were caspase10 primer (Gene bank, AB247499.1) and the 18S rRNA primer (Gene bank, EF126037.1). 18S rRNA was used as the internal standard control, and the expression level for 18S rRNA was quantified using the 2−ΔΔCt method (Pfaffl, 2001). In addition, an 18S rRNA and caspase10 primer of olive flounder was designed using the Primer3 program (Rozen & Skaletsky, 2000). The primers used for the analysis were as follows: caspase10 forward (5’-GCACATGGACATCCTGAGTG-3’) and caspase10 reverse (5’-AGGCTGCTCATTTCACTGCT-3’), 18S rRNA forward (5’-ATGGCCGTTCTTAGTTGGTG-3’), and 18S rRNA reverse (5’-CACACGTGATCCAG TCAGT-3’).
All experiments were performed in triplicate for enhanced accuracy. The data were expressed as mean±SE. Statistical analysis of the data was performed using the statistical software package R-3.0.1 (Okorie et al., 2013). Significant differences in the data values were confirmed using the one-way analysis of variance (ANOVA). The significance test of expression among the individuals was conducted using the Duncan’s least significant range (LSR) test, when p<0.05.
RESUlTS
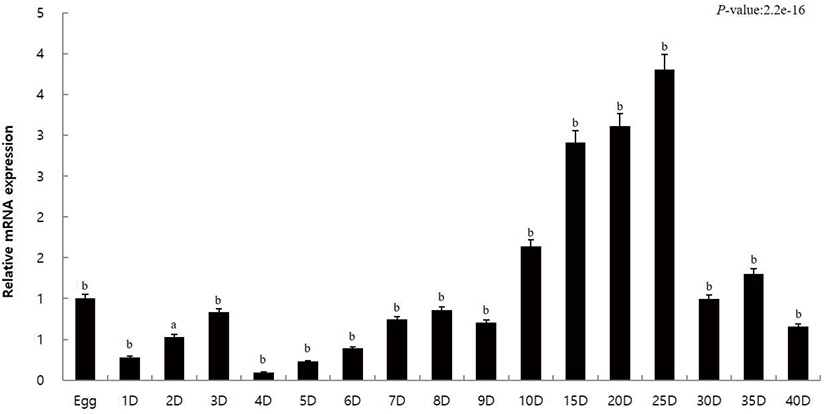
The innate immune response of caspase10 was investigated through the expression analysis of caspase10 during the initial developmental stages of olive flounder to 40 DAH. Quantitative analysis was performed using qRT-PCR. Using gene-specific primer for caspase10, the amount of mRNA expression in each step was calculated using the 18S rRNA. The results were compared with eggs (the value set to 1) to investigate the expression during different developmental stages. The expression of caspase10 compared to that in eggs (1.0-fold) was low until 9 days (0.7-fold) after hatching (Fig. 1). It gradually increased from day 10 (1.6-fold) and showed high expression on day 25 (3.8-fold) (Fig. 1). A dip in the expression profile of caspase10 was reported after day 25 (Fig. 1).
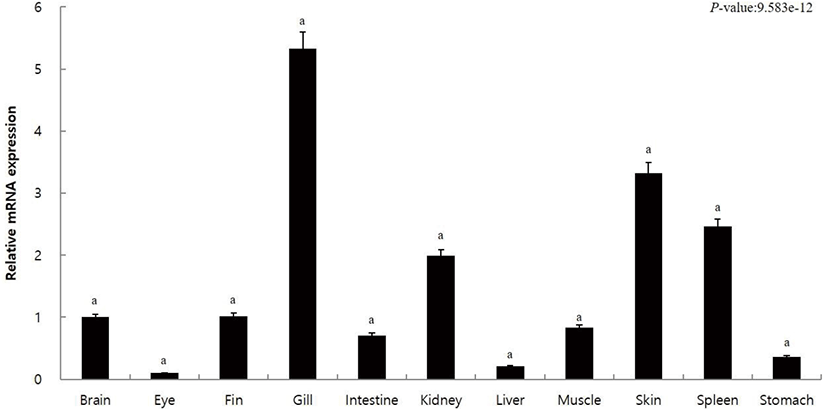
In order to observe the expression of caspase10 in the different tissues of olive flounder, we extracted the brain, eye, fin, gill, intestine, kidney, liver, muscle, skin, spleen, and stomach tissues. The qRT-PCR analysis using gene-specific primer for caspase10 was performed to examine the tissue-specific expression of caspase10. The relative mRNA expression in each tissue was calculated using 18S rRNA, and the same was compared with the expression within the brain (the value set to 1). Results revealed high expression of capsase10 in the kidney (2.0-fold), spleen (2.5-fold), and skin (3.3-fold) and the highest expression in the gills (5.3-fold) (Fig. 2). The expression of caspase10 was low in the liver (0.2-fold), stomach (0.4-fold), intestine (0.7-fold), muscle (0.8-fold), fin (1.0-fold), and brain (1.0-fold), and the lowest in the eye (0.1-fold) (Fig. 2).
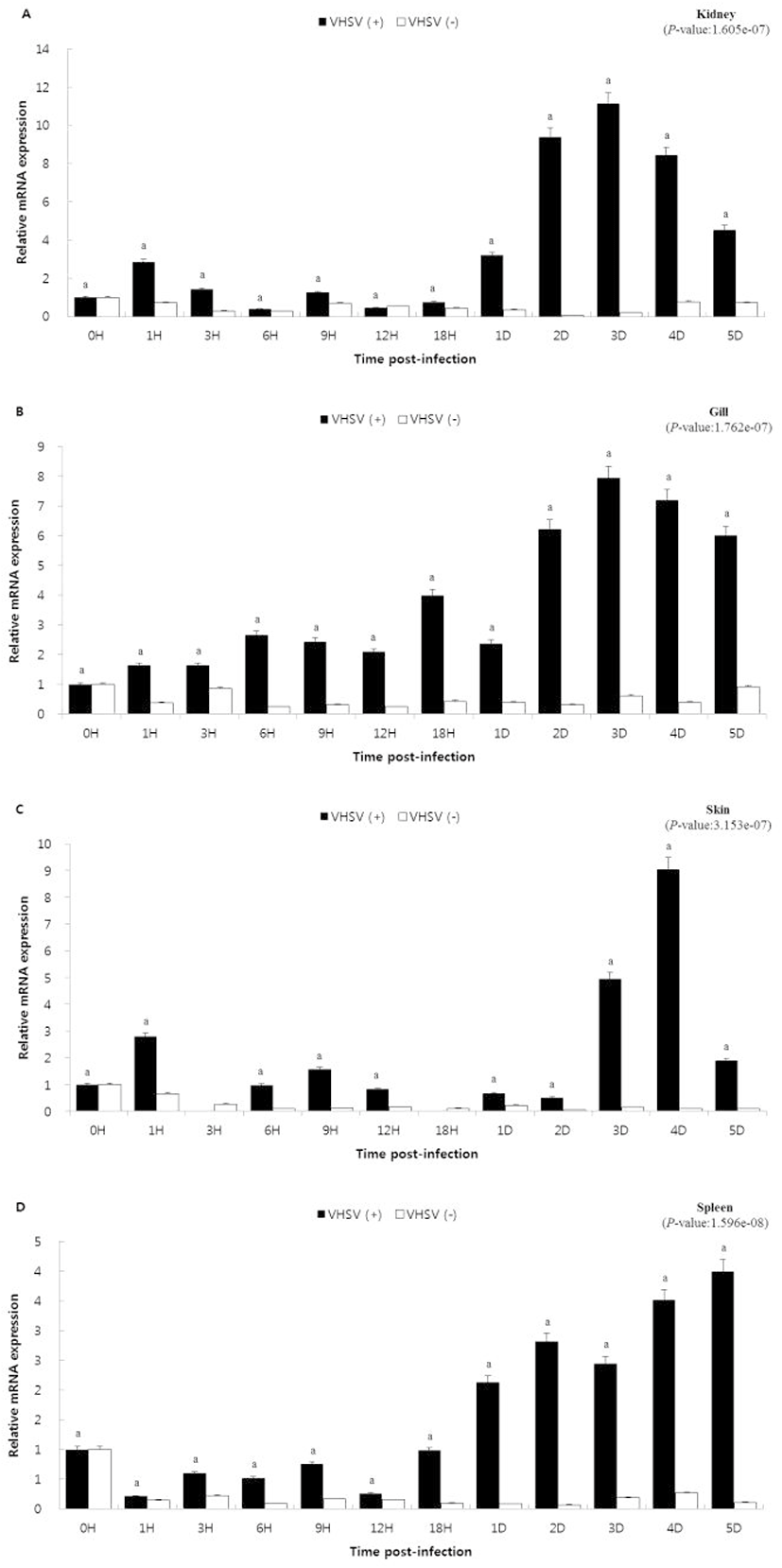
In order to study the role of caspase10 in the immune response to viral infection, caspase10 gene expression was examined over time in olive flounder artificially infected with VHSV. The relative mRNA expression for each hour was calculated using the reference gene, olive flounder 18S rRNA, and the specific expression was examined by comparing the result with 0 h (the value set to 1), that is, the start time of infection. In the kidney and gills, caspase10 showed similar expression patterns. The expression increased from day 2 (kidney, 9.4-fold; gill, 6.2-fold) showing the highest expression on day 3 (kidney, 11.1-fold; gill, 7.9-fold) and then again decreased from day 4 (kidney, 8.4-fold; gill, 7.2-fold). In the skin, caspase10 did not show significant expression until 2 days (0.5-fold) after hatching. After that, the expression increased on day 3 (4.9-fold) and day 4 (9.0-fold), and thereafter again decreased on the day 5 (1.9-fold). In the spleen, the expression of caspase10 gradually increased from day 1 (1.0-fold) and showed the highest expression on day 5 (4.0-fold).
DISCUSSION
The olive flounder is an economically important aquaculture fish species. However, in the recent years, disease causing infections have led to a significant damage of the fish species (Kim et al., 2009). Like most fishes, olive flounder lives in an aquatic environment from its egg stage and hence is exposed to many pathogens from the time of fry. In mammals, immune systems, such as the bone marrow and lymph nodes are less evolved, but they produce antibodies and destroy the microorganisms by the innate immune responses (Magnadottir, 2006). Therefore, in order to understand the innate immune response of olive flounder, we observed the tissue-specific expression of caspase10 during the different developmental stages of olive flounder, that is, from egg to day 40 after hatching.
The expression pattern of caspase10 was similar to that of eggs until day 9 after hatching, but the expression increased on day 10 and showed high expression on day 25 after hatching (Fig. 1). Day 28 after hatching marks the late metamorphosis in olive flounder (Kim, 2009). During this stage the stomach becomes enlarged, and the digestive tract develops into a structure similar to that of adult fish. The clear demarcation of the front, back, and rectum marks the transition to the fry or young stage. The changes that resemble olive flounder to an adult fish are accompanied by an increase in the caspase10 expression. However, the caspase10 expression decreased 25 DAH (Fig. 1). The variation in the caspase10 expression indicates that the expression is higher when the fry olive flounder enters the bottoming stage after the metamorphosis is completed, rather than in the early of metamorphosis. As a result, caspase10 is expected to be deeply involved in immune response of olive flounder, as during the late stages of development, the formation of the immune specific tissues is completed.
Fish gills, skin, and stomach are considered as the peripheral lymphoid tissues, and the kidneys and spleen are the main lymphoid tissues that proliferate and differentiate into lymphocytes (Sizemore et al., 1984; Zapata & Cooper, 1990; Rombout et al., 1993; Petrie-Hanson L & Ainsworth AJ, 2001; Langenau et al., 2004). These study revealed high caspase10 expression in the lymphoid tissues such as the gill, spleen, and kidney of olive flounder (Fig. 2). According to a previous report, high expression of caspase10 has been reported in the blood, gills, and spleen of stone rock black rockfish, in the gills and spleen of snakehead murrel, and in the white blood cells (WBC), gills, and spleen of olive flounder (Kurobe et al., 2007; Arockiaraj et al., 2013; Elvitigala et al., 2015). From these results, it could be confirmed that despite the differences depending on the type of fish, most of them show high caspase10 expression in immune tissues.
Caspases, based on their primary functions are classified into inflammatory, initiator, and effector caspases. Caspase10 is an initiating caspase of the cysteine-aspartic acid protease family and plays an important role in regulating cell death or apoptosis. In this study, the expression of capase10 was observed in response to VHSV infection, one of the major causes of mass death of olive flounder. The kidney being an immune-related organ is rich in leukocytes and other immune cells. On VHSV infection, kidney exhibited no significant difference in caspase10 expression. However, a heightened expression was reported on day 3 which again decreased thereafter (Fig. 3A) (Uribe et al., 2011). A similar caspase10 expression pattern was observed in the gills and skin, which remains exposed to the aquatic environment and thus pose as the most convenient invasion route for numerous microorganisms (Fig. 3B and 3C) (Kim, 2012). In the case of spleen, the caspase10 expression gradually increased over time and showed the highest expression on day 5 (Fig. 3D) (Sizemore et al., 1984; Rombout et al., 1993). Therefore, it can be expected that the high expression of caspase10 in the immune system-related tissues appropriately contributes to cell death during viral infection. Interpreting the results of the study, it could be stated that the expression of caspase10 is not just limited to viral invasion, but gradually increases as the disease becomes severe, promoting the induction of apoptosis and subsequent killing of the infected cells. In this study, the expression of caspase10 was analyzed to study the immune system of fish. The expression of caspase10 under viral infection suggested the possibility of killing infected cells in an immune-related tissue, which otherwise could be the cause of death in a virus-infected fish. These results are expected to be useful as the preliminary data for studying the immune responses in fish.