INTRODUCTION
Calcium is a highly multifaceted intracellular messenger that controls a wide range of cellular functions by regulating the activity of numerous target proteins. Calcium functions by binding directly to a target protein, detecting changes in calcium concentron, and then activating various downstream reactions (Berridge, 2014). During mammalian fertilization, phospholipase C zeta (PLCZ), a factor from sperm, induces repetitive changes in the intracellular calcium concentration [Ca2+]i, termed [Ca2+]i oscillations. These [Ca2+]i oscillations are responsible for the initiation of fertilization event including, cortical granule exocytosis to block to polyspermy, resumption of meiosis, recruitment of maternal mRNAs and activation of embryonic genome, and further embryonic development (Ajduk et al., 2008). Once PLCZ were released into egg, PLCZ triggers activation of phosphoinositide (PI) pathway that produces IP3 and 1, 2-diacylglycerol (DAG) by hydrolysis of PIP2. Increase intracellular inositol 1,4,5, tri phosphate (IP3) concentration is in charge for mediating Ca2+release from endoplasmic reticulum (ER) via IP3 receptor (IP3R) (Miyazaki et al., 1993; Malcuit et al., 2006; Yoon & Kang, 2011 ). The pattern of [Ca2+]i oscillations in fertilized mouse eggs affects blastocyst formation and developmental competence (Ozil et al., 2006). The each of cellular event of egg activation or fertilization is controlled by a different number of [Ca2+]i oscillations Experiments altering extracellular [Ca2+] concentration in the middle of Ca2+ oscillations affected both the refilling rate and the rate of Ca2+ influx, which is in determining the intervals of Ca2+ transients. (Takahashi et al., 2013). The sources of [Ca2+]i elevation during [Ca2+]i oscillations are Ca2+release from ER through IP3R and Ca2+ ion influx through Ca2+ channel on the plasma membrane. Ca2+ channels on the plasma membrane have been investigated in hormone sensitive cell, neurons, and skeletal and heart muscle cells and characterized into voltage-dependent Ca2+ channel (VDCCs), ligand-gated [Ca2+]i channel, and leak-channel. VDCCs expressed on muscle cell or neuron is specified into L, T, N, P, Q, and R type VDCCs by threshold or their sensitivity to peptide toxins isolated from cone snails and spiders (Bourinet & Zamponi, 2017). It has been shown that plasma membrane potentials of mammalian oocyte are changed according to their maturational stage from germinal vesicle stage to meiosis II stage that could be fertilized by sperm (Lee et al., 2004). Also, addition of VDCCs blockers inhibits mammalian oocyte maturation or embryo development (Petr et al., 1997; Hotsuliak et al., 2002). Summarizing these reports, the Ca2+ required for mouse egg maturation appears to have been separated by Ca2+ influx, but the Ca2+ origin of Ca2+ oscillations that appear during fertilization have not been reported.
The present study was aimed to investigate that the effect of extracellular Ca2+ on Ca2+ oscillation and Ca2+ influx through VDCCs during fertilization. Also, we investigated the localization of N and P/Q type voltage dependent calcium channel in mouse eggs and the role in fertilization and further embryonic development by using N type VDCCs specific inhibitor.
MATERIALS AND METHODS
Five to six weeks old ICR or C57BL/DBA F1 hybrid female mouse were purchased from Samtako (Seoul, Korea). Female mice were superovulated by 5 IU pregnant mare serum gonadotropin (PMSG; Lee Biosolution, Maryland Heights, MO, USA), followed by injection with 5 IU human chorionic gonadotropin after 48 h (hCG, Sigma-Aldrich, St. Louis, MO, USA). Cumulus enclosed mature eggs were collected from oviduct at 14 h post hCG. Cumulus cells were removed with 0.1% hyaluronidase (Sigma-Aldrich) in HEPES-buffered Tyrode’s lactate solution (TL-Hepes) medium (Parrish et al., 1988). All experiments described in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of CHA University and performed in accordance with the guidelines presented by IACUC (Approval No. IACUC170141, IACUC200157).
[Ca2+]i changes were measured by loading eggs with 1 μM Fluo-4 AM or fura-2-acetoxymethyl ester (Molecular Probes, Eugene, OR, USA), a [Ca2+]i sensitive indicator dye, supplemented with 0.02% pluronic acid (Molecular Probes) in TL-HEPES, and monitored under an Axiovert 200 M microscope fitted with a ×10 objective and CCD camera controlled with AxioVision software 4.8.1 (Carl Zeiss, Jena, Germany). [Ca2+]i was monitored by measuring fluorescence from individual eggs loaded on a temperature-controlled chamber dish (SEC, Seoul, Korea) coated with CellTak® (BD Biosciences, San Jose, CA, USA). Microinjection of porcine sperm factor (pSF: prepared as in Kurokawa et al., 2005) or 10 μM adenophostin A (Ad A, Cayman Chemical, Ann Arbor, MI, USA) was performed using a Nikon (Narishige, Japan) manipulator with a picoinjector (Femtojet, Effendorf, Hamburg, Germany) via pneumatic pressure (Kim et al., 2011). Thapsigargin (TG, 10 μM, EMD Biochemicals, Gibbstown, NJ, USA) was employed for assessment of the releasable [Ca2+]i content in eggs, and strontium chloride (10 mM SrCl2) was added in [Ca2+]i free TL-HEPES medium. 2.5 μM Latrunculin A (Lat A, Calbiochem, San Diego, CA) were applied for actin polymerization inhibitor that modifies the organization of the egg cortex and inhibit [Ca2+]i oscillation (Lee et al., 2016).
Immunostaining of eggs was carried out as described in a previous report (Kim et al., 2011). In briefly, zona pellucida were removed with acid-Tyrode’s solution (pH 2.5, Sigma-Aldrich), and fixed in 4% paraformaldehyde and 0.1% Tritone X-100 for 30 min at 4°C. Eggs were blocked in protein block (Dako, Glostrup, Denmark) for 2 h at 4°C, followed by incubation with an anti-CACNA1B (CaV2.2) antibody for N-type VDCC or anti-CACNA1B (CaV2.1) antibody for P/Q type VDCC (1:100) for overnight. Eggs were stained with Alexa Fluor 555 goat anti-rabbit IgG (1:200, Molecular Probes), FITC-phalloidin (Molecular Probes), and DAPI (Molecular Probes), and mounted in VECTASHELD mounting medium (Vector Laboratories, Burlingame, CA, USA). Mounted eggs were observed with using a laser scanning confocal microscope with the Z-stack (LSM 880, Carl Zeiss, Germany). The primary antibody were omitted for negative control. For immunostaining of blastocysts, blastocysts were treated with acid-Tyrode’s solution to remove zona pellucida, and fixed in 4% paraformaldehyde and 0.1% Tritone X-100 for 30 min at 4°C. Immunofluorescence analysis was performed for inner cell mass (ICM) with anti-oct-¾ antibody and trophectoderm (TE) with actin-phalloidin and total cell number with DAPI.
Sperm was collected from epididymis of 12-week-old male mice in HTF supplemented with 5% KnockOut™ Serum Replacement (KSR, Gibco, Grand Island, NY, USA) and capacitated for 30 min. Capacitated spermatozoa were added (1–2×106/ mL) to eggs in HTF medium and incubated for 6 h with or without ω-Conotoxin CnVIIA, a specific Blocker of N-Type [Ca2+]i Channels (Alomone Labs, Jerusalem, Israel). Fertilization rate was calculated as percentage of eggs transforming into two pronuclei (2PN) at 8 h post-insemination. Blastocyst formation rate was calculated as percentage of 2PN transforming into blastocysts. The quality of blastocysts was analyzed on the basis of morphological characteristics and with immunofluorescence analysis.
All the results were originated from at least three independent experiments. All statistical analyses were performed using Student’s t tests with GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). p-values lower than 0.05 or 0.001 were considered statistically significant.
RESULTS
To investigate the effect of extracellular Ca2+ ion on [Ca2+]i oscillation, we tested Ca2+ free medium with porcine sperm factor(pSF). pSF is a strong [Ca2+]i oscillation inducer in mouse eggs. However, in Ca2+ free medium, [Ca2+]i oscillations were abolished completely (Fig. 1A). Another [Ca2+]i oscillation inducer, Adenophostin A (Ad A) is a potent IP3R agonist which is much more potent than IP3. Microinjection of Ad A, also showed strong [Ca2+]i oscillation, but not in Latrunculin A (Lat A) contained medium (Fig. 1 B). Lat A, known as actin filament disruptor, also has the ability to inhibit Ca2+ influx. To analyze Ca2+ influx activity of Lat A, we induced store-operated Ca2+ entry (SOCE) using thapsigargin (TG) and Ca2+. After Ca2+ store depletion by TG, addition of 5 mM CaCl2 into medium induces Ca2+ influx from extracellular environment. Lat A treatment inhibited Ca2+ influx with 5 mM CaCl2 (Fig. 1C). The above results indicate that the [Ca2+]i oscillation that appears during fertilization is introduced from the extracellular environment, which is through the Ca2+ channel.
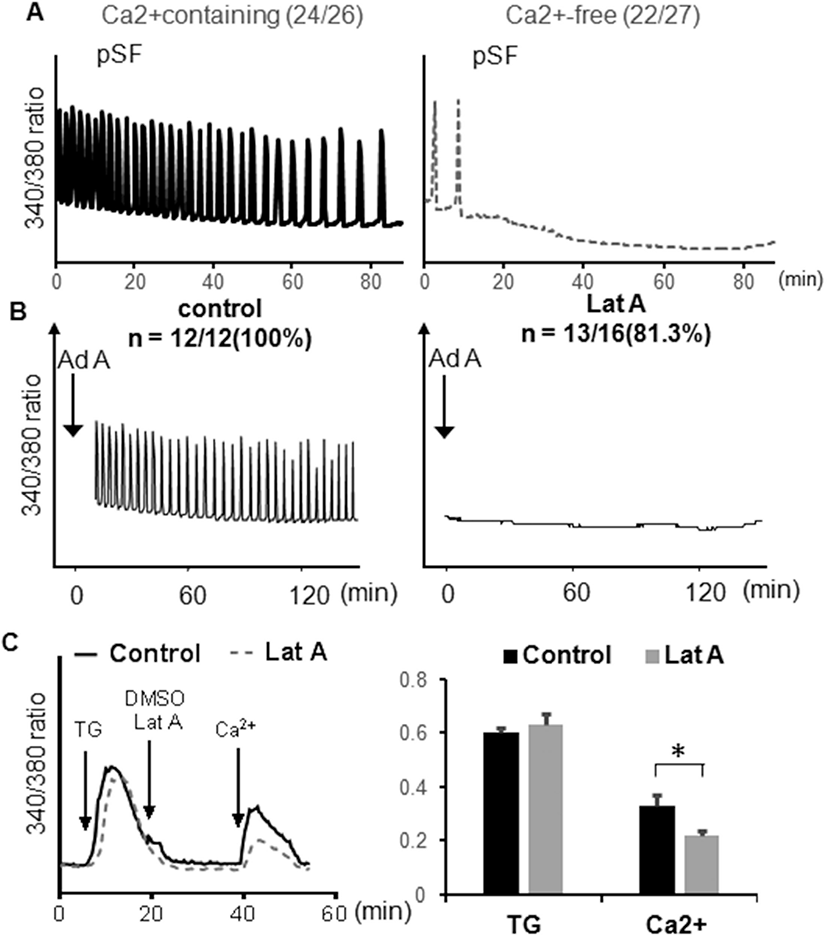
The distribution of N-type and P/Q type VDCCs were examined in mouse eggs using antibody, localized on the plasma membrane of anti-CACNA1B (CaV2.2) antibody for N-type VDCC or anti-CACNA1B (CaV2.1) antibody for P/Q type VDCCs. Mature metaphase II eggs contained both VDCCs in the cortex, and little staining was observed in the interior of the eggs. Both VDCCs were not observed near spindle and cortical granule free domain (Liu, 2011). Also, VDCCs on the cortex were organized clusters, 1–2 μm in diameter (Fig. 2, yellow arrow). Some clusters of P/Q type VDCCs in interior of the eggs were more than 3 μm in diameter (Fig. 2, yellow arrow head).
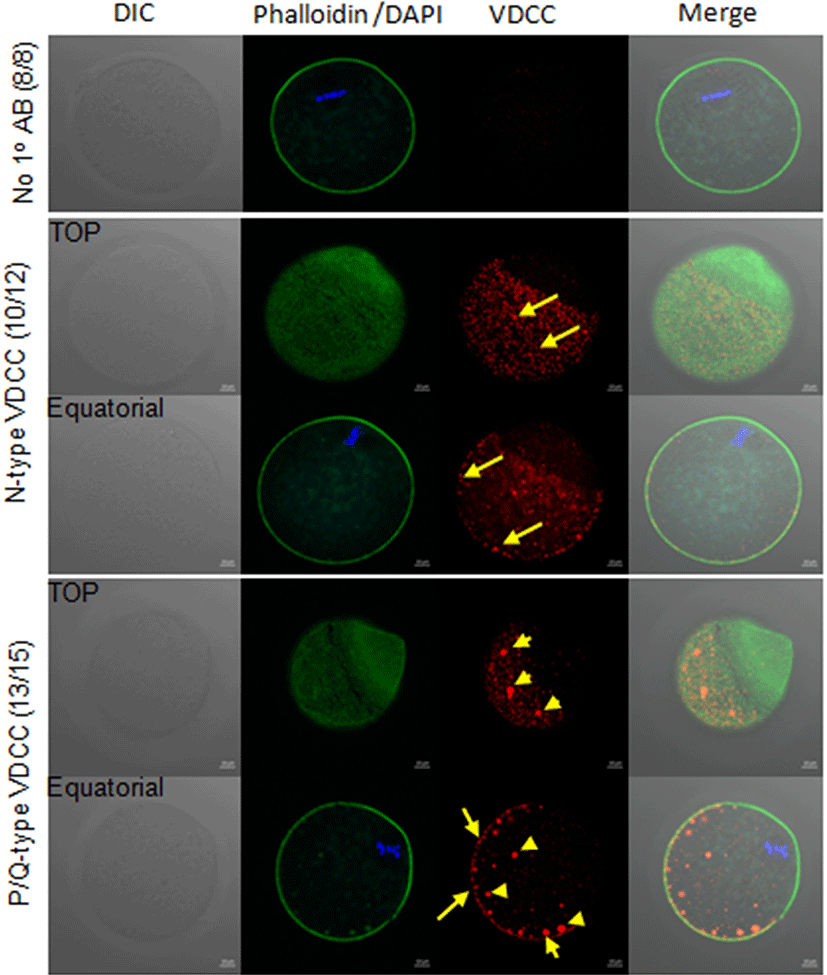
To eliminate the potential effects of VDCC inhibitors on sperm fertility including, sperm motility, acrosome reaction, we used SrCl2 for egg activation during [Ca2+]i monitoring. In 10 mM SrCl2 in [Ca2+]i free CZB medium, eggs showed average 3–4 spikes per 90 min, however, in ω-Conotoxin CnVIIA containing medium, 21 out of 31 eggs showed 6–7 spikes/90 min 10 out of 31 eggs showed less than 3 spikes / 90 min (Fig. 3A). In analytic data of [Ca2+]i oscillation pattern, there are no statistical difference in intracellular Ca2+ concentration, basal level, the amplitude of spike (Max-min), and duration of spikes, but the number of spikes per 90 min represented higher in ω-Conotoxin CnVIIA compare to control.
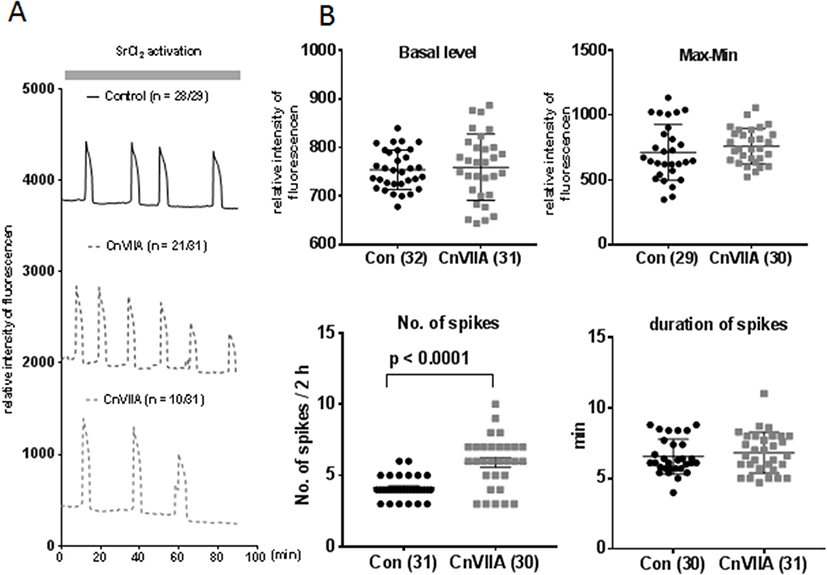
Mature eggs were preincubated in 1 μM ω-Conotoxin CnVIIA for 1 hr in HTF medium and fertilized with capacitated sperm in same medium. Two pronucleus zygotes were collected at 8 h post fertilization. There is no difference in fertilization rate (2PN/MII eggs, Fig. 4A and B), but blastocyst formation were inhibited in ω-Conotoxin CnVIIA compare to control (BL/2Cell).
DISCUSSION
[Ca2+]i serves as a second messenger in many signal transductions. In hormone-sensitive cells, neurons, muscle, and many other cells that respond to extracellular signals, Ca2+ serves as a second messenger to triggers intracellular response (Conrard & Tyteca, 2019). Free Ca2+ cytoplasmic concentration is kept very low at resting state 10–100 nM, in comparison to the ER / sarcoplasmic reticulum (60–500 nM) and the extracellular medium or serum (1.8 mM) (Conrard & Tyteca, 2019). The voltage dependent Ca2+ channels (VDCCs) activates on membrane depolarization and mediates Ca2+ influx in response to action potentials and subthreshold depolarization signals (Catterall, 2011). Also, VDCCs plays an essential role in mammalian oocyte maturation (Lee et al., 2004). There are at least six pharmacologically distinct calcium channels types, including L-, N-, P/Q-, T, and R-type calcium channels (Catterall, 2000). The mammalian genome encodes as many as ten different genes that lead to different types of Cav subunits, the principal pore forming subunit that forms the core of the calcium permeable ion channel, and have been grouped into three major class such as Cav1, Cav2, and Cav3. Four different L-type VDCCs were encodes in Cav1. Cav1–3 encodes P/Q, N, and R-type VDCCs respectively. The last Cav3 family represents T-type VDCCs (Bourinet & Zamponi, 2017). Various peptide toxins extracted from marine snails (conotoxins) and spiders (agatoxins, grammotoxin and DW13.3 Filistata hibernalis) are known to potently inhibit the activity of certain voltage-dependent calcium channels (Bourinet & Zamponi, 2017). The ω-conotoxin used in this study is a small peptide ranging in size from 13 to 30 amino acids, and are selective for N-type VDCCs. ω-conotoxin is by blocking the channel pore, which is accomplished by tight binding of the toxin to channel pore (Ramirez et al., 2017).
In this study, we investigated the effect of extracellular Ca2+ on [Ca2+]i oscillation during fertilization. Porcine sperm factor is a strong inducer of [Ca2+]i oscillation in mouse eggs (Kurokawa et al., 2007). It has higher frequency of [Ca2+]i oscillation mouse sperm in mouse eggs (Kurokawa et al., 2005). However, it could not induce [Ca2+]i oscillation in Ca2+ free medium except the initial Ca2+ spikes (Fig. 1A). Also, a non-hydrolyzable analogue of IP3, adenophostin A (Ad A) is a strong inducer [Ca2+]i oscillation. It could produce a Ca2+ release from intracellular Ca2+ store, such as ER ( Jellerette et al., 2004). In this study, Ad A could not induce [Ca2+]i oscillation in [Ca2+]i free condition (Fig. 1 B). These results support that [Ca2+]i oscillation during fertilization necessities the extracellular Ca2+ ion. Latrunculin A (Lat A) has been known as an actin polymerization inhibitor, which disrupted intracellular [Ca2+]i channels on the ER, inositol 1,4,5 triphosphate receptors (Wakai et al., 2012). In this study, Lat A inhibited [Ca2+]i influx by store operated [Ca2+]i influx (Fig. 1C). These results represented that the [Ca2+]i oscillation during fertilization needed the [Ca2+]i influx through [Ca2+]i channel on the plasma membrane.
External [Ca2+]i can enter cells through channels and/or transporters. The T-type voltage-gated [Ca2+]i channel (CaV 3.2) (Kang et al., 2007) was one of the first characterized channels in mouse eggs, although its function is still unclear because female mutant mice lacking CaV 3.2 are fertile (Chen et al., 2003). To investigate whether VDCCs other than the T-type VDCCs are involved in mouse egg fertilization, immunofluorescence staining was performed using P/Q and N-type VDCCs antibodies. In addition, in the P/Q type, fluorescent staining of patches with a diameter of 3 μm or more was observed in the eggs cytoplasm (Fig. 2). Next, inhibitor of N-type VDCCs, ω-Conotoxin CnVIIA interrupted [Ca2+]i oscillation during fertilization. The pattern of [Ca2+]i oscillation during fertilization was known as key regulator of further embryonic development (Ozil et al., 2006). Egg activation comprised a temporal serious of [Ca2+]i dependent events, that include cortical granule exocytosis, cell cycle resumption with change of MPF/MAP kinase activities, and recruitment of maternal mRNAs (Ducibella et al., 2002; Ajduk et al., 2008). In this study, we used 10 mM SrCl2 to induce Ca2+ oscillation and to eliminate the effect of VDCCs inhibitor on sperm physiology (Rahban & Nef, 2020). The [Ca2+]i oscillation pattern of ω-Conotoxin CnVIIA is different compared to the control. Most of the eggs showed a higher frequency than control or less frequency of [Ca2+]i oscillation (Fig. 3B). And those eggs showed a low amplitude of [Ca2+]i spike at end of [Ca2+]i oscillation. These results indicate a reduction in [Ca2+]i retention by ω-Conotoxin CnVIIA. Last, we investigated the effect of inhibition N-type VDCCs using ω-Conotoxin CnVIIA on embryonic development. There is no statistic differences in fertilization, but blastocyst formation was inhibited by ω-Conotoxin CnVIIA treatment during fertilization. In the Fig. 3, we did not observe long term [Ca2+]i monitoring, but most of the eggs showed a decrease of amplitude of [Ca2+]i spike. Compared to the control, it can be seen that [Ca2+]i oscillations were induced to stop prematurely, and the signal transduction required for sufficient egg activation eventually failed (Ducibella et al., 2002). Also, functional inhibition of VDCCs induced abnormal [Ca2+]i oscillation during fertilization or egg activation process might be the reason of poor embryogenesis (Fig. 4).
In the present study, we confirmed that VDCCs were present in ovulated mouse eggs. Ca2+ influx is essential for Ca2+ oscillations during mammalian fertilization. This Ca2+ influx may be controlled through the N- or P/Q-type voltage dependent Ca2+ channel. Functional inhibition of the P/Q-type VDCC requires further investigation. Abnormal VDCC expression in eggs should be tested in fertilization failure or low fertilization eggs in infertile/subfertile women.
