INTRODUCTION
In particular, many traits revealed during the development of fish provide important basic data for clarifying the taxonomic relationships of allied species (Blaxter, 1974; Balon, 1985). In the study of the systematics traits of fish of the Cypriniformes during the development, the characteristics of the fertilized eggs were found as demersal egg and epipelagic eggs even within the Squalidus same genus. Melanophore vesicles were formed after hatching (AH) for short fishes with a hatching time of 1–2 days. At the end of the egg development, melanophore vesicles are developed in the eye of the embryo. This will be used as a systematics trait to grasp the taxonomic relations of the genus Squalidus (Song et al., 2017).
Fish belonging to Cypriniformes, Cobitoidei, and balitorid are distributed in Europe and Asia (Kottelat, 2012; Nelson et al., 2016). Balitorid fish living in Korea includes Orthrias toni, O. nudus, and Lefua costata (Kim, 1997; Kim et al., 2005; Kim & Park, 2007), of which L. costata is distributed in Korea, Japan, and the Maritime Province of Siberia in Russia (Kim, 1997; Kim et al., 2005).
The genus Lefua fish in Korea are morphologically similar to the fish of the same genus in Japan. They show distinguishable characteristics that appeared in early life history. This study is to investigate the differences in the characteristics of genus Lefua fish between Korea and Japan.
Genus Lefua fish living in Japan are 5 species including L. costata, L. nikkonis, L. echigonia, L. torrentis, and L. tokaiensis (Hosoya, 2019). In case of L. torrentis, previous research by Aoyama & Doi (2011) distinguished the morphological differences of the head and the distribution location of melanocyte appearing in the early life period. In addition, it was found that L. sp. lives in the same water stream as L. echigonia but L. echigonia lives in wetland areas and L. sp. in mountainous valley areas. Later, L. sp. was reported as a new species L. torrentis by Hosoya et al. (2018).
Record of early life history for L. costata only been recorded Uchida (1939). It is necessary to clearly observe the characteristics of the early life history that appear during the development.
Therefore, this study aims to observe the early life history, such as egg development and fish larvae morphology of L. costata and compare the results with those of related species to use them as basic data for taxonomic studies.
MATERIALS AND METHODS
The broodstocks used in the study were collected using a stake net at Sanguncheon located in Sonyang-myeon, Yangyang-gun, Gangwon-do in June 2018, and were transported to the laboratory.
Housed in a square water tank (1×1×1 m), the broodstocks were bred in a raceway culture, and frozen mosquito larvae were supplied twice/day as feed (Hikari, Japan), and the water temperature was 22°C–24°C (mean 23±1°C).
For broodstocks, females had a total length of 7–9 cm and a weight of 4–5 g (n=10), and males had a total length of 5–7 cm and a weight of 2–3 g (n=20). In order to induce natural spawning, males and females were anesthetized with an anesthetic (MS-222, Ethyl 3-aminobenzoate methanesulfonate, Sigma-Aldrich, St. Louis, MO, USA). Then a sexual hormone for maturity (LHRH, Syndel, Nanaimo, Canada) was injected into the muscle on the basis of 0.5 mL per kg. In order to stimulate the water temperature, the breeding of water temperature in the tank was raised from 23°C–25°C (mean 24±1°C).
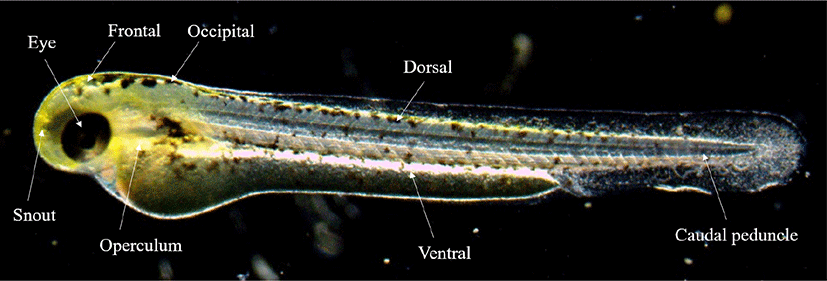
The fertilized eggs, the rearling of water temperature was maintained at 24°C–26°C (mean 25±1°C). In order to observe the egg development, 30 eggs were randomly selected and observed with a stereomicroscope (JP SMZ800, Nikon, Tokyo, Japan), and up to 0.01 mm was measured using a universal projector ( JP V-12BM, Nikon). For the food of fish larvae, nauplius larvae of Artemia sp. were supplied from 15 days after hatching (DAH). From then on, the dry feed (350 μm, Jeilfeed, Daejeon, Korea) was kneaded and supplied. In order to describe the morphological characteristics from juvenile fish which were selected, the external morphology of five was observed with a stereoscopic microscope immediately AH, and the size was measured up to 0.01 mm with a universal projector. We followed the study of Okiyama (1988) for the criteria for each developmental stage of fish larvae. Ontogenetic patterns of the melanophore was observed by selecting 8 sites for each growth stage (Fig. 1).
RESULTS
The female spawning amount was about 900–1,200 eggs (mean 1,050±150). The eggs were a light green color with completely circle shape, and were adherent demersal eggs with adhesiveness on the surface. The egg sizes were 1.15–1.27 mm (mean 1.21±0.06 mm, n=30).
At 30 minutes after fertilization (AF), blastodisc was formed on the upper part of the egg yolk (Fig. 2A), and it reached the 2-cell stage as the cleavage occurred in the animal pole in 1 hour AF (Fig. 2B). At 1 hour 30 minutes AF, it reached the 4-cell stage as the meridional cleavage occurred in the same size (Fig. 2C). At 2 hours AF, it reached the 8-cell stage in the same manner (Fig. 2D), and reached the 16-cell stage at 2 hours 30 minutes AF (Fig. 2E). At 3 hours AF, it reached the 32-cell stage as the number of cell divisions increased (Fig. 2F), and at 3 hours 30 minutes AF, it reached the 64-cell stage (Fig. 2G). At 4 hours AF, it reached the loss stage as the number of cells increased beyond count (Fig. 2H). At 6 hours 30 minutes AF, it reached the blastula stage (Fig. 2I), and at 9 hours AF, it reached the early gastrula stage as covering down from the animal pole to the vegetal pole (Fig. 2J). At 10 hours 30 minutes AF, half of the 50% was covered and it reached the middle gastrula stage (Fig. 2K), and at 12 hours AF, 90% was covered and it reached the late gastrula stage (Fig. 2L). At 14 hours AF, the blastopore was closed and an embryonic body was formed (Fig. 2M). At 19 hours AF, the optic vesicle was formed, 10 myotomes were formed, and Kuffer's vesicle was formed (Fig. 2N). At 30 hours AF, Kuffer's vesicle was lost. As the tail became longer, it began to separate from the egg yolk, and blood flow was observed from the heart along the egg yolk (Fig. 2O). At 33 hours AF, the tail lengthened to the upper part of the head, and the movement of the embryonic body became active (Fig. 2P). At 42 hours AF, melanocyte began to deposit in the eyes, and hatching started as they broke through the egg membrane from the head (Fig. 2Q). At 48 hours AF, more than 50% of the fertilized eggs were hatched (Fig. 2R).
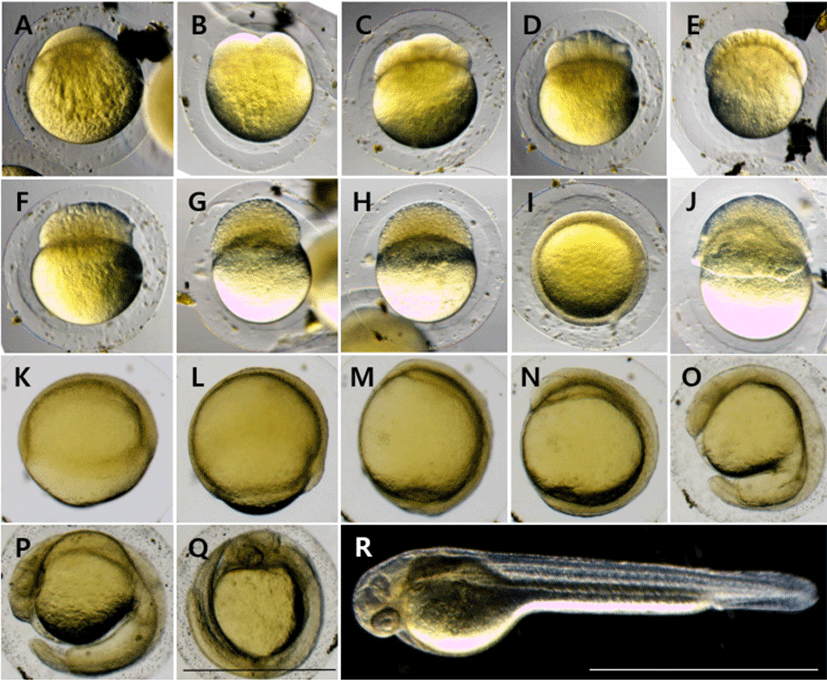
| Stage | Elapsed time | Characters | Fig. 2 |
|---|---|---|---|
| Cell cleave period | |||
| Blastodisc | 0 h 30 min | Blastodisc is formed | A |
| Two cell | 1 h 00 min | 2–1 array of blastomeres | B |
| Four cell | 1 h 30 min | 2–2 array of blastomeres | C |
| Eight cell | 2 h 00 min | 2–4 array of blastomeres | D |
| Sixteen cell | 2 h 30 min | 4–4 array of blastomeres | E |
| Thirty-two cell | 3 h 00 min | 4–8 array of blastomeres | F |
| Sixty-four cell | 3 h 30 min | 8–8 array of blastomeres | G |
| Morula | 4 h 00 min | The size of the blastomere is getting smaller | H |
| Blastula | 6 h 30 min | The surface of the blastomere coincides with the egg yolk | I |
| Gastrulation 1/3 | 9 h 00 min | Covered 1/3 of egg yolk | J |
| Gastrulation 2/3 | 10 h 30 min | Covered 2/3 of egg yolk | K |
| Gastrulation 3/3 | 12 h 00 min | Covered 3/3 of egg yolk | L |
| Embryonic period | |||
| 14 h 00 min | Development of embryo | M | |
| 19 h 00 min | Development of Kuffer’s vesicle | N | |
| 30 h 00 min | Appearence of otocyst and long tail | O | |
| Embryo just before hatching | 33 h 00 min | The movement of the embryonic body was fastered | P |
| Hatching period | 42 h 00 min | Hatching started | Q |
| 48 h 00 min | Hatching rate 50% | R |
The larvae immediately AH are 2.70–2.93 mm in total length (mean 2.81±0.11 mm, n=5), their mouth and anus were not open, had egg yolk, and the entire body was covered with membrane fins. Melanocytes were deposited in the eyes, and the body was lying sideways on the floor with no swimming ability, and the movement of the tail was observed (Fig. 3A).
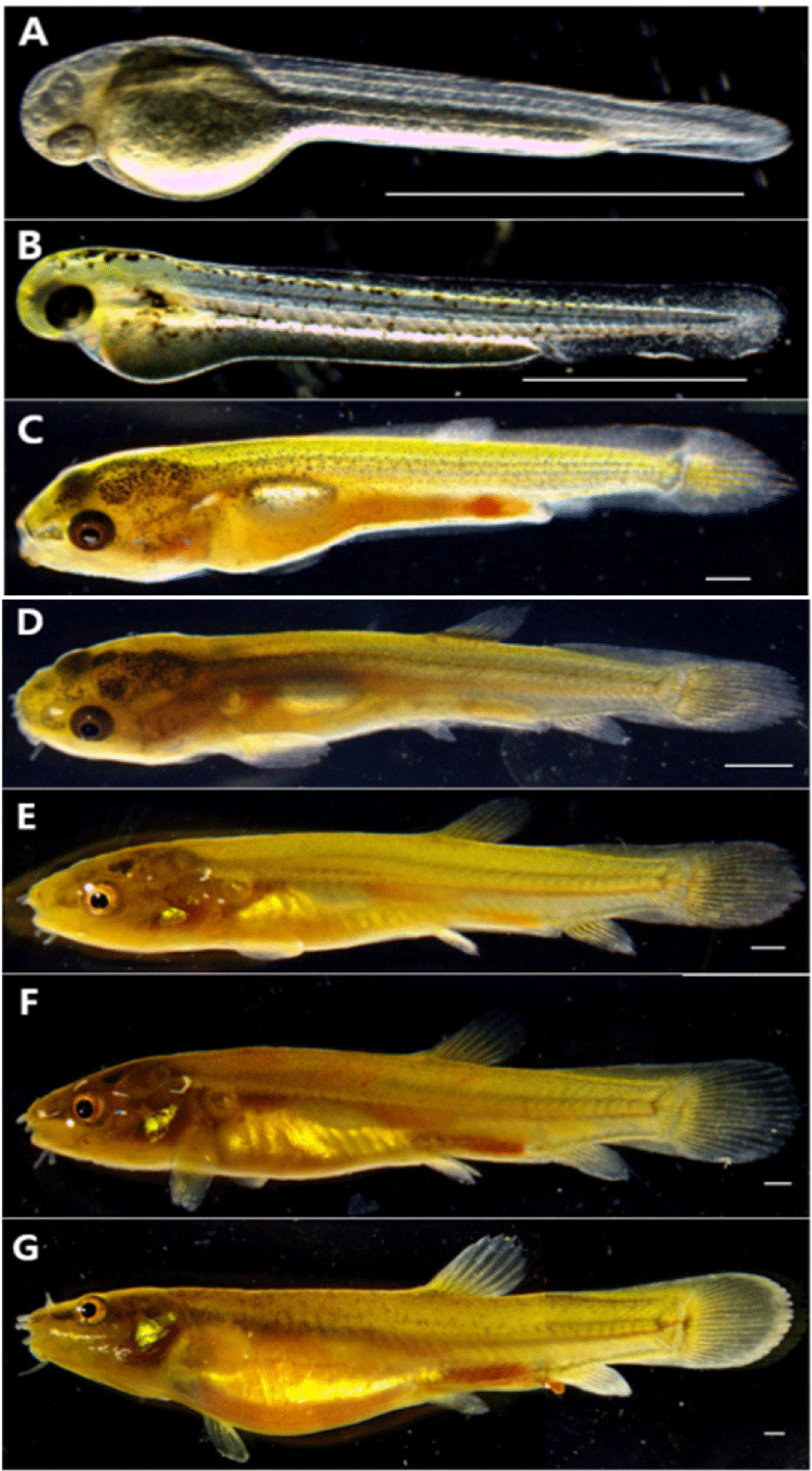
On the 1st day AH, the egg yolk-sac larvae was 3.71–3.87 mm in total length (mean 3.79±0.08 mm, n=5), and twig-shaped melanocyte was deposited on the upper part of the head, body, and the upper part of the egg yolk. The melanocyte deposited on the eyes became darker, and the anus began to open (Fig. 3B).
On the 8th day AH, the postflexion larvae are 8.97–9.90 mm in total length (mean 9.43±0.46 mm, n=5), 7 fin rays were developed in the differentiated dorsal fins, and 4 fin rays were developed as the anal fins begins to differentiate. The distal part of the notochord was bent 45° to the upper part, and 16 fin rays were developed in the caudal fins. The medial fin was in the form of a non-differentiated membrane fin, and the dorsal, anal and caudal fins had developed girdle but were connected by a membrane. The melanocyte deposited on the body became spot-shaped and mostly disappeared in the body part, and some remained in the head and the front part of the body (Fig. 3C).
On the 15th day AH, the postflexion larvae are 12.2–13.8 mm in total length (mean 13.0±0.80 mm, n=5), and the number of fin rays for each part increased to 9 dorsal fins and 6 anal fins, and 3 fin rays were formed in ventral fins. Membranous dorsal, anal, caudal, and ventral fins were all separated, and the caudal fins were made of a membrane from the caudal part to the middle part of fin rays. In the abdomen, one air bladder in the shape of a cylinder was divided into two, which repeated staying stationary and swimming in the middle layer. Two pairs of barbels were formed around the mouth (Fig. 3D).
On the 20th day AH, the postflexion larvae are 14.8–16.3 mm in total length (mean 15.5±0.75 mm, n=5), and the number of fin rays for each part increased to 12 dorsal fins, 10 anal fins and 17 caudal fins, and 3 fin rays were formed in ventral fins. The melanocyte was scattered in the shape of small spots on the upper part around the upper part of the gill and the side line (Fig. 3E).
On the 26th day AH, juveniles are 17.7–19.4 mm in total length (mean 18.5±0.85 mm, n=5), and the number of fin rays for each part reached a whole number, and the number of barbels increased to 4 pairs, and the length of the barbels was shorter than the ones located in the middle between the top and bottom (Fig. 3F).
On the 31st day AH, juveniles are 21.5–23.2 mm in total length (mean 22.3±0.85 mm, n=5), and the fin rays for each part were Ⅳ8 dorsal fins and Ⅲ8 anal fins, and the melanocyte in the shape of irregular small spots along the middle of the lateral line of the body formed a horizontal band, and the outer shape was the same as that of broodstocks (Fig. 3G).
DISCUSSION
The eggs of L. costata are demersal eggs with adherent properties and exhibited the same properties as M. mizolepis, Kichulchoia multifasciata, Koreocobitis naktongensis belonging to Cobitidae (Kim et al., 1987; Kim & Lee, 1995; Song et al., 2009). The size of the eggs is 1.15–1.27 mm (mean 1.21±0.06 mm, n=30), which is similar to mean 1.12 mm of M. mizolepis (Kim et al., 1987), Cobitid fish and smaller than mean 2.50 mm of K. multifasciata (Kim & Lee, 1995). Immediately AH, the larvae were 2.70–2.93 mm in total length (mean 2.81±0.11 mm, n=5), and Uchida (1939) recorded the total length as 3.90 mm. Given that the back of the head is connected to the end of egg yolk with the membrane fin, and melanocyte is not deposited on other areas except the eyes, the morphological characteristics were not significantly different from the results of this study.
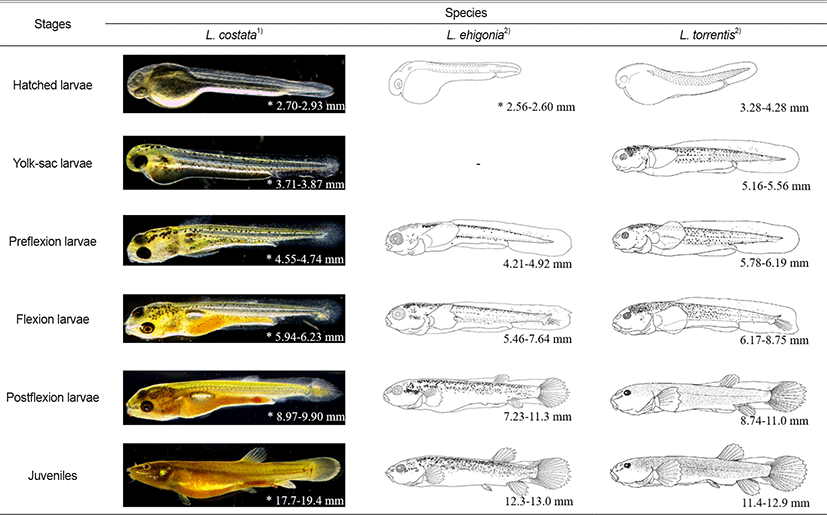
In this study, we compared the morphological characteristics of the larvae of L. costat with its related species (eg. Japanese L. echigonia and L. torrentis). The body lengths of hatching larvae of L. echigonia and L. torrentis are 2.56–2.60 mm and 3.28–4.28 mm, respectively, which are larger than L. echigonia but smaller than L. torrentis, showing the middle size of the two species (Table 2). The melanocyte of hatching larvae of L. costata was generally deposited in the eyes, but that of L. echigonia, and L. torrentis were not deposited. The egg yolk larvae of L. costata was 3.71–3.87 mm, and twig-shaped melanocyte was deposited in the upper part of the head, back and even the top of the egg yolk and tails. L. torrentis was 5.16–5.56 mm in body length, and twig-shaped melanocyte was deposited in the upper part of the head, back and even the top of the egg yolk and tails, and the distribution position was similar to that of L. costata and L. torrentis had an egg yolk, an open mouth, and developed pectoral fins, whereas L. costata during a similar period did not have an open mouth nor developed pectoral fins, showing a morphological difference during the egg yolk larvae period.
TL. total length; BL, body length.
The preflexion larvae of L. costata were 4.55–4.74 mm in total length, and the melanocyte deposited in the upper part of the head was deposited more widely. During this period, their mouth was open and the pectoral fins developed. The preflexion larvae of L. echigonia are 4.21–4.92 mm in body length, and the upper part of the head had the spot-shaped melanocyte which is smaller than that of the twig-shaped melanocyte deposited in the entire body. The preflexion larvae of L. torrentis were 5.78–6.19 mm in body length, and both small spot-shaped and twig-shaped melanocytes were deposited in the upper part of the head, so the distribution location of the melanocyte during the preflexion larvae period showed an intermediate form between L. costata and L. echigonia.
The flexion larvae of L. costata are 5.94–6.23 mm in total length, and the size of the melanocyte deposited in the trunk and the upper part of the egg yolk decreased, and the notochord distal end of the tail part began to bend to 45°, and fin rays were developed in the caudal fin. The flexion larvae of L. echigonia are 5.46–7.64 mm in body length, and the size of small spot-shaped melanocyte on the upper part of the head increased, and it was widely deposited along the upper part of the gills and the middle of the body. The flexion larvae of L. torrentis are 6.17–8.75 mm in body length, and the melanocyte deposited on the upper part of the head was deposited to a similar size, and the distribution range of the melanocyte in the middle and anterior part of the body became wider. The flexion larvae of L. costata had a similar form of the melanocyte deposited on the upper part of the gills to that of L. torrentis, and the flexion larvae of L. echigonia were widely distributed, showing a difference.
The postflexion larvae of L. costata are 8.97–9.90 mm in body length, and the size of the twig-shaped melanocyte deposited on the upper part of the head decreased, and the fins of each part were developed, and fin rays were formed as the dorsal and anal fins developed. The postflexion larvae of L. echigonia are 7.23–11.3 mm in body length, and the melanocyte deposited on the upper part of the gills, the middle of the body, and the dorsal side was deposited more widely, and fin rays were formed on the dorsal and anal fins. The postflexion larvae of L. torrentis are 8.74–11.0 mm in body length, and the melanocyte deposited on the upper part of the head, the middle of the body, and the dorsal side became smaller in the shape of small spots, and was deposited all over the body except for the back of the snout and the upper part of the gill. The developmental pattern of melanocytes during the postflexion larvae period was similar to that of L. torrentis. During this period, the membranes connected to the dorsal, anal and caudal fins did not disappear, which was the same as that of closely related species.
Juveniles of L. costata are 17.7–19.4 mm in total length, and the shape of the melanocyte was similar to that of adult fish. One pair of barbels was developed in the nostrils and three pairs of barbels around the mouth. Juveniles of L. echigonia are 12.3–13.0 mm in body length, and most of the melanocyte was irregularly deposited on the upper half of the body. One pair of barbels was developed in the nostrils and three pairs of barbels around the mouth. Juveniles of L. torrentis are 11.4–12.9 mm in body length, and small spot-shaped melanocyte was evenly deposited all over the body. One pair of barbels was developed in the nostrils and three pairs of barbels around the mouth. In the case of L. costata and closely related species, the fins connected by the membrane were all separated into the dorsal, anal and caudal fins as migrating to the juvenile period, and the number of barbels was the same (Fig. 4).
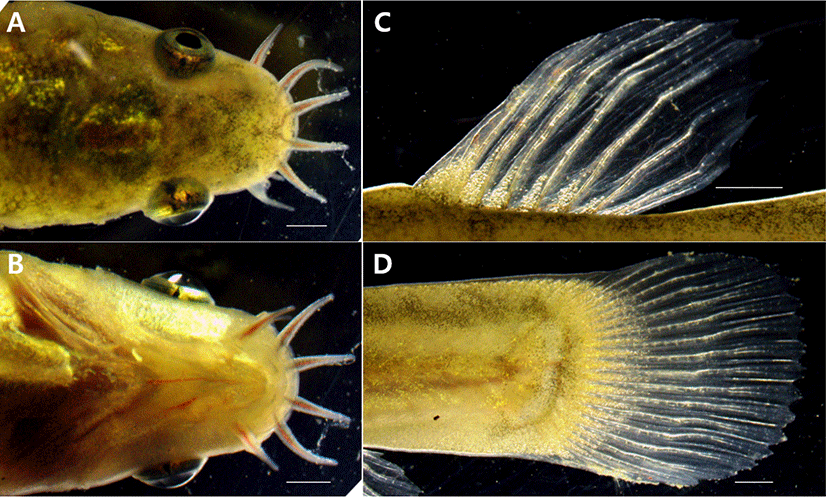
Aoyama & Doi (2011) suggested the external morphological differences between the two species because L. echigonia’s eye size is larger than the length from mouth to nostrils, and L. torrentis has an eye size smaller than the length from mouth to nostrils. L. costata’s eye size (0.003–0.005 mm) is similar to the length from mouth to nostrils (0.003–0.005 mm), showing a difference from L. echigonia, and L. torrentis.
When observed from the back side, the cornea of the eye is off the head in the L. costata’s head, and the same shape was also seen when observed from the bottom. When observed from the back and the bottom, the L. echigonia’s cornea was outside the head, while the cornea was located inside the head in L. torrentis, and the head shape of L. costata was similar to that of L. echigonia. Ito et al. (2019) compared the distribution of melanocytes in the dorsal and caudal fins including the head shape with the closely related species and reported L. tokaiensis as a new species. When comparing the result with that of L. costata, L. tokaiensis has the melanocyte deposited on its dorsal and caudal fin, and the dark band is formed at the starting point of the caudal fin. L. echigonia has the distribution pattern of melanocytes which is similar to that of L. tokaiensis, but showed a difference because there was no dark band, and melanocytes and dark bands were not observed on the dorsal and caudal fins. L. costata was similar to L. torrentis among the closely related species as no melanocyt and dark bands were observed on the dorsal and caudal fins (Fig. 5).
According to the results of the study, L. costata’s head shape, size of hatching larvae showed the intermediate shape between Japanese L. echigonia and L. torrentis, and the distribution locations of melanocytes were found to vary according to the growth stage of fish larvae, showing differences from the closely related species, thus providing basic data for taxonomic studies.
