INTRODUCTION
There are 189 genera and 1,395 species of Gobiidae fish (order Perciformes) worldwide, with 19 genera and 39 species reported in South Korea (Nelson et al., 2016).
Favonigobius gymnauchen is a fish species belonging to the Gobiidae genus Favonigobius and is distributed around Jeju Island, the West and South Sea coasts and estuaries in South Korea, and China, Japan, and the Western Pacific (Chae et al., 2019).
Studies on F. gymnauchen include its ecology, feeding habits (Huh & Kwak, 1998), histology (Kim et al., 2016), reproductive cycles (Lee et al., 2000), spawning behavior, and morphological development of eggs, larvae, and juveniles (Jin et al., 2003).
Studies of the skeletons of Gobiidae fish cover Chaenogobius laevis (Kim & Han, 1989), Periophthalmus modestus (Lee, 1990), Luciogobius guttatus (Kim et al., 1992), L. grandis (Yun et al., 2020), Tridentiger obscurus (Hwang et al., 2018), and T. trigonocephalus (Han et al., 2018).
Studies of the skeletal development of larvae and juveniles handle the systematic basic traits of the young stage (Mook, 1977; Potthoff et al., 1987, 1988; Potthoff & Tellock, 1993; Liu, 2001; Sfakianakis et al., 2004; Ҫoban et al., 2009). These studies also provide important data for species identification and the skeleton characteristics of broodstock. Hence, the specific and systematic study of F. gymnauchen is also required (Koumoundouros et al., 1997a, b).
Many Gobiidae fishes, including F. gymnauchen, coexist and reproduce in coastal estuaries and brackish waters where freshwater and seawater meet. This can make it difficult to identify the larvae and juveniles of these species, which often have similar external morphological characteristics. Systematic research data are needed to identify interspecies differences during the larval and juvenile periods. Therefore, the aim of this study was to observe the skeletal development of larval and juvenile F. gymnauchen inhabiting South Korea and compare it with other Gobiidae fish species to provide basic data for systematic research.
MATERIALS AND METHODS
Specimens were obtained using the larval and juvenile samples of Jin et al. (2003), which were stained with Walker & Kimmel’s staining method (2007), and preserved in KOH 0.1% and glycerol 50%. After the staining was completed, the larvae and juveniles were observed, and sketches were drawn for each part using a stereoscopic microscope (SMZ800, Nikon, Tokyo, Japan). The name of each part of the skeleton was given in accordance with Han et al. (2018).
RESULTS
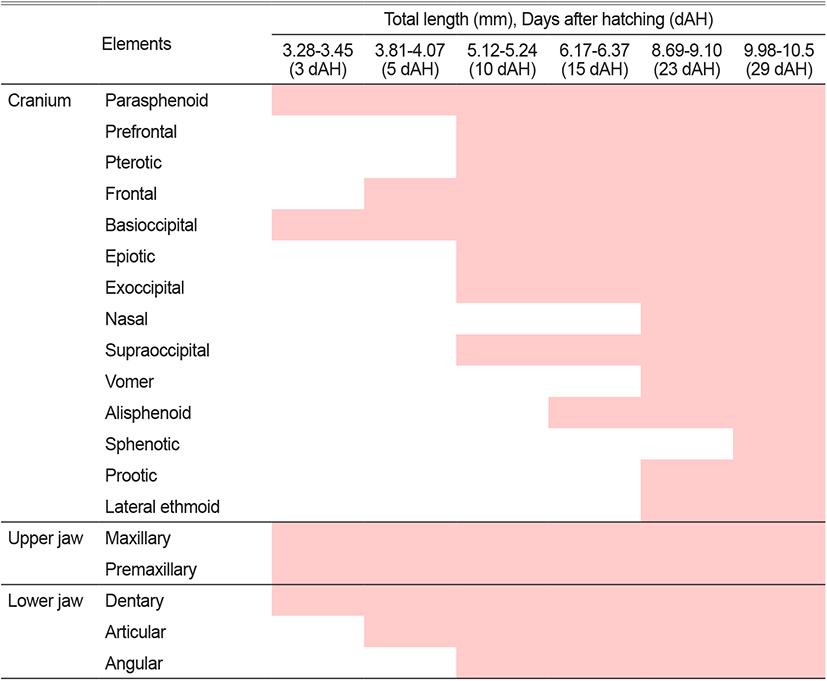
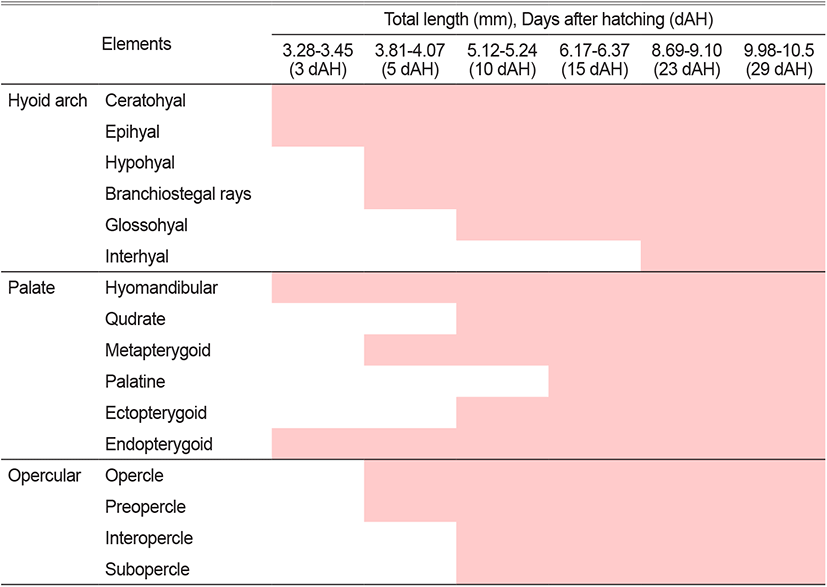
The total length (TL) of larvae 3 days after hatching (DAH) were 3.28 to 3.45 mm (mean 3.34±0.12 mm, n=10), with a line-shaped parasphenoid ossification in the cranium and basioccipital ossification in the back. The premaxillary and maxillary bones were ossified in the upper jaw, and the dentary bone was ossified in the lower jaw. The hyomandibular and endopterygoid in the palate and ceratohyal and epihyal in the hyoid arch were ossified (Fig. 1A).
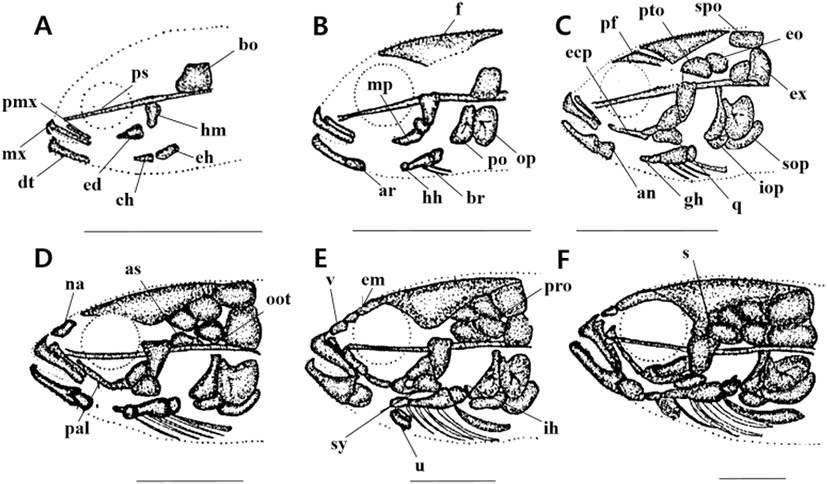
The TL of 5 DAH larvae were 3.81 to 4.07 mm (mean 3.96±0.18 mm, n=10), with frontal ossification in the cranium. The metapterygoid in the palate and preopercle and opercle in the opercular were ossified. The articular in the lower jaw and the hypohyal in the hyoid arch were also ossified (Fig. 1B).
The TL of 10 DAH larvae were 5.12 to 5.24 mm (mean 5.15±0.08 mm, n=10), and the pterotic, epiotic, exoccipital, prefrontal, and supraoccipital in the cranium were ossified. The subopercle and interopercle in the opercular, and the angular in the lower jaw were ossified. The glossohyal and four branchiostegal rays in the hyoid arch, and the ectopterygoid and quadrate in the palate were also ossified (Fig. 1C).
The TL of 15 DAH larvae were 6.17 to 6.31 mm (mean 6.21±0.09 mm, n=10). The opisthotic, alisphenoid, and nasal were ossified in the cranium, while the palatine in the palate and the six branchiostegal rays in the hyoid arch were all ossified (Fig. 1D).
The TL of 23 DAH larvae were 8.69 to 9.10 mm (mean 8.87±0.28 mm, n=10), with ossifications of the prootic, vomer, and ethmoid in the cranium. In the hyoid arch, the urohyal and interhyal were ossified, and the symplectic was ossified in the palate (Fig. 1E).
The TL of 29 DAH juveniles were 9.98 to 10.5 mm (mean 10.2±0.36 mm, n=10), and the ossification of the cranium and visceral skeleton was completed by ossifying the sphenotic in the cranium (Fig. 1F).
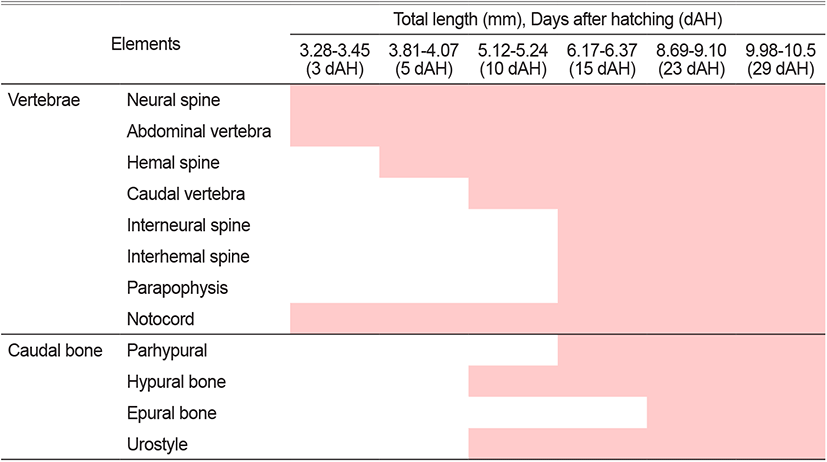
The mean TL of 3 DAH larvae was 3.34 mm. In the vertebrae, three neural spines were ossified from the front of the abdominal vertebrae in the centrum form (Fig. 2A).
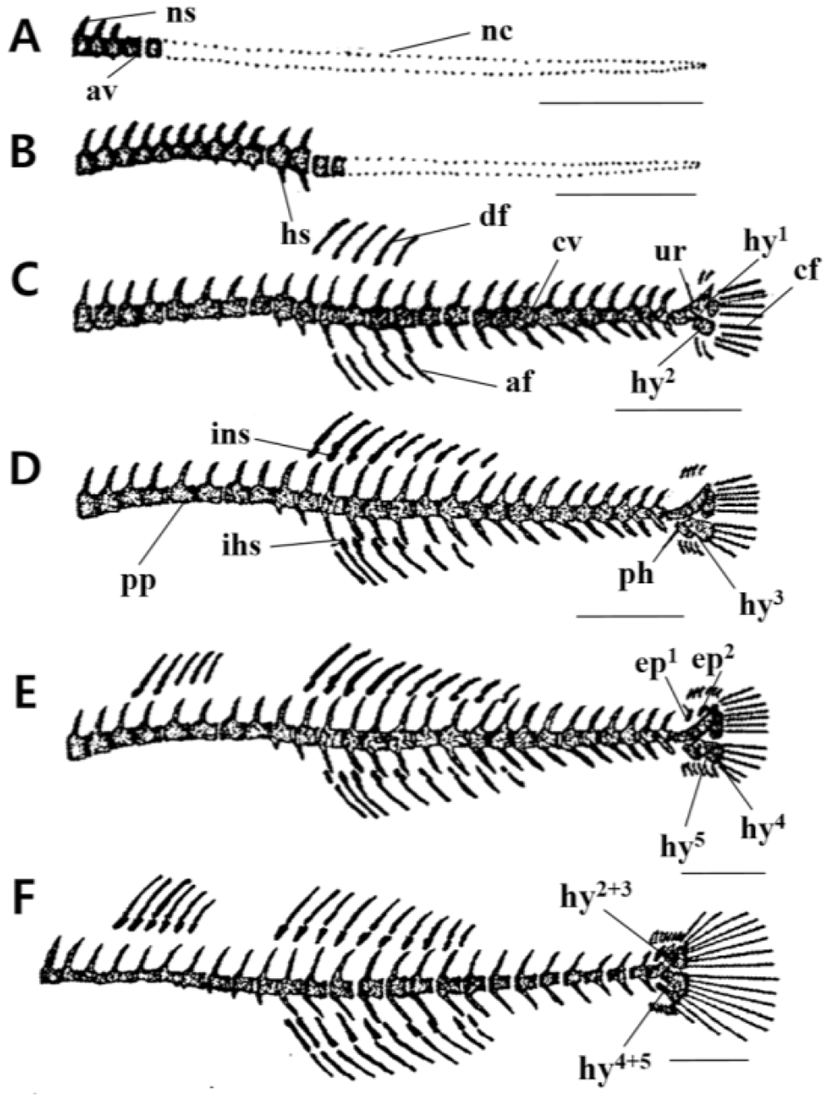
With a mean TL of 3.96 mm for 5 DAH larvae, ten abdominal vertebrae and four caudal vertebrae began to ossify from the front of the vertebrae, and the number of neural spines increased to 13 (Fig. 2B).
The 10 DAH larvae had a mean TL of 5.15 mm, with the number of caudal vertebrae increasing to 15. The urostyle and two hypural bones in the lower part also began to ossify (Fig. 2C).
The 15 DAH larvae had a mean TL of 6.21 mm, with the ossification of four interneural spines supporting the second dorsal fin and four hemalneural spines supporting the anal fin. At the lower part of the urostyle, one parhypural was ossified, and the number of hypural bones increased to three (Fig. 2D).
With a mean TL of 8.87 mm for 23 DAH larvae, two interneural spines supporting the first dorsal fin and the interneural spines supporting the second dorsal fin increased to ten. The number of hemalneural spines supporting the anal fin increased to nine, two epural bones were ossified at the top of the urostyle, and the number of hypural bones increased to five (Fig. 2E).
The 29 DAH juvenile had a mean TL of 10.2 mm. The number of interneural spines in the first dorsal fin increased to six, 26 (10+16) vertebrae were ossified, and the hypural bones were combined into three bone fragments (1, 2+3, 4+5) (Fig. 2F).
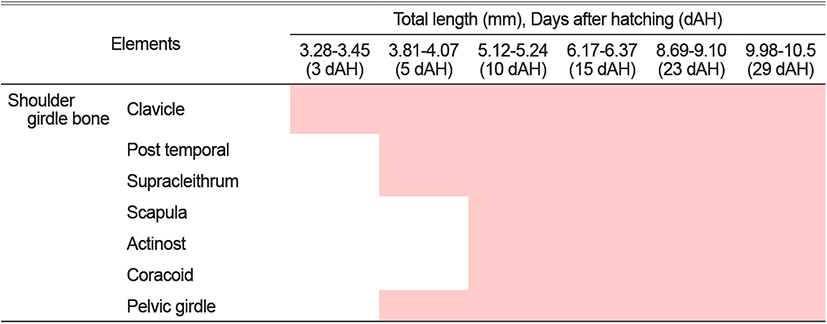
The 3 DAH larvae had a mean TL of 3.34 mm, and the line-shaped clavicle behind the gill started to be ossified in the pectoral girdle bone (Fig. 3A).
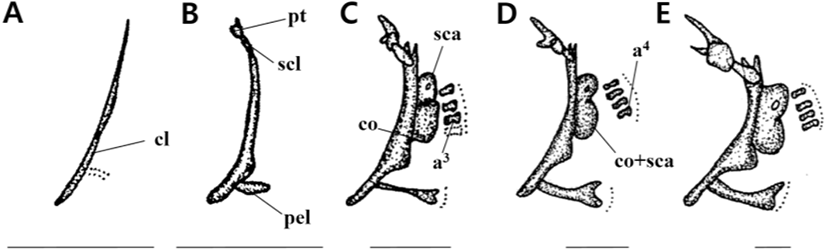
The 5 DAH larvae had a mean TL of 3.96 mm, with supraclavicular ossification. The post temporal bone was ossified at the top, and the pelvic girdle began to ossify at the bottom of the clavicle (Fig. 3B).
With a mean TL of 5.15 mm for 10 DAH larvae, the scapula and coracoid were ossified in the clavicle’s back, and three actinosts were ossified in the lower part of the pectoral fin. The ventral fins began to develop in the pelvic girdle (Fig. 3C).
The 15 DAH larvae had a mean TL of 6.21 mm, with the ossification of four actinost bones and the formation of rays at the back of the pelvic girdle (Fig. 3D).
The 23 DAH juveniles had a mean TL of 8.87 mm. The pectoral girdle’s skeleton was completed as the scapula and coracoid were ossified. The pelvic girdle also fully supported the ventral fin as its ossification was completed (Fig. 3E).
DISCUSSION
The skeleton development of F. gymnauchen started from the three DAH with a mean TL of 3.34 mm. The premaxillary, maxillary, and dentary bones were first developed to help with feeding. The hyomandibular and endopterygoid in the palate linked to the jaw skeleton, and the ceratohyal and epihyal in the hyoid arch were ossified to complete the skeleton of 29 DAH juveniles with a mean TL of 10.2 mm. As F. gymnauchen grew, the bones involved in its feeding and respiratory functions were preferentially ossified.
Luciogobius guttatus began to develop its skeleton at 11 DAH with a mean TL of 5.50 mm; the skeleton was completed at 47 DAH, with a mean TL of 13.4 mm (Kim et al., 1992). Luciogobius grandis started to develop its skeleton at three DAH with a mean TL of 4.01 mm; its skeleton was completed at 36 DAH with a mean TL of 12.2 mm (Yun et al., 2020). Tridentiger obscurus began to develop its skeleton at eight DAH with a mean TL of 8.62 mm and its skeleton was completed at 52 DAH with a mean TL of 18.2 mm (Hwang et al., 2018). The skeleton development of T. trigonocephalus started at seven DAH with a mean TL of 4.44 mm and was completed at 40 DAH with a mean TL of 13.3 mm (Han et al., 2018). Chaenogobius laevis started to develop its skeleton at 9–10 DAH with a mean TL of 6.00 mm and finished its skeleton development at 43 DAH with a mean TL of 14.2 mm (Kim & Han, 1989). F. gymnauchen completed the development of its skeleton earlier than these other species.
The cranium is an essential skeleton element for feeding and respiration, and is the part in which skeleton development preferentially occurs (Vandewalle et al., 1997), since the clavicle, which supports the sternohyoideus muscle, plays an essential role in mouth opening and feeding (Matsuoka, 1987; Wagemans & Vandewalle, 1999).
The clavicle of F. gymnauchen started to develop in a thin line-shape at 3 DAH with a mean TL of 3.34 mm, and developed in connection with the cranium at 23 DAH with a mean TL of 8.87 mm. The age and size when the clavicle became connected to the cranium in other species was 29 DAH with a mean TL of 11.4 mm for L. grandis (Yun et al., 2020), 25 DAH with a mean TL of 10.4 mm for C. laevis (Kim & Han, 1989), 25 DAH with a mean TL of 7.60 mm for L. guttatus (Kim et al., 1992), 28 DAH with a mean TL of 9.32 for T. trigonocephalus (Han et al., 2018), and 52 DAH with a mean TL of 18.3 for T. obscurus (Hwang et al., 2018). F. gymnauchen and L. guttatus (Kim et al., 1992) showed similarities in the connection period of the clavicle with the cranium and the mean TL values. In F. gymnauchen, the clavicle was connected to the cranium earlier than L. grandis (Yun et al., 2020), T. trigonocephalus (Han et al., 2018), and T. obscurus (Hwang et al., 2018). Chaenogobius laevis (Kim & Han, 1989) showed a similar period for the connection but there was a difference in size, as it was smaller than F. gymnauchen.
The period when the cranium was connected to the clavicle in Gobiidae fish developed at the end of the postflexion larvae period. During this period, the pelvic girdle development led to a demersal life, and this development pattern is believed to be related to the swimming function for demersal life.
In F. gymnauchen’s pterygiophore and vertebrae, the abdominal vertebrae began to develop together with the neural spine in a state of notochord with an incomplete skeleton in three DAH larvae (mean TL of 3.34 mm). At 15 DAH with a mean TL of 6.21 mm, there were developments of a lateral process in the abdominal vertebrae, an interneural spine on the dorsal side, and an interhemal spine on the ventral side.
At 8 DAH L. grandis with a mean TL of 4.87 mm, the neural spine began to ossify in the state of the notochord; the interneural spine and interhemal spine were ossified after vertebrae and fin rays developed at 36 DAH (with a mean TL of 12.2 mm) (Yun et al., 2020).
In T. trigonocephalus (Han et al., 2018) and T. obscurus (Hwang et al., 2018), the neural spine was developed after the start of the ossification of the abdominal vertebrae. The interneural spine and interhemal spine were also ossified after the development of the vertebrae and fin rays.
The abdominal vertebrae and neural spine in F. gymnauchen were ossified simultaneously, completing the ossification of the pterygiophore after the development of the vertebrae. Such development patterns were the same as those of T. obscurus (Hwang et al., 2018) and T. trigonocephalus (Han et al., 2018). On the other hand, the neural spine was ossified earlier than the abdominal vertebrae in L. grandis (Yun et al., 2020), C. laevis (Kim & Han, 1989), and L. guttatus (Kim et al., 1992). The earlier development of the fin rays than the vertebrae showed there were differences in development even within the family Gobiidae.
When the development of the vertebrae and fins was completed, the development of the pterygiophore seemed to be related to increasing the driving force in the fishes’ swimming ability (Lee et al., 2001). In addition, in the early larval period of F. gymnauchen, larvae lacked the power to swim independently, but it seemed that feeding and swimming became active along with the development of the fin rays.
The development of vertebrae in F. gymnauchen proceeded from the anterior abdominal vertebrae to the posterior caudal vertebrae, and this developmental trend was the same in other Gobiidae species. The urostyle bone of F. gymnauchen developed with the ossification of the vertebrae. The urostyle bone was ossified before the ossification of the vertebrae in L. grandis (Yun et al., 2020). The urostyle bone was ossified after the ossification of the vertebrae in T. obscurus (Hwang et al., 2018) and T. trigonocephalus (Han et al., 2018). In other words, there were diverse developmental patterns depending on the species. When comparing the number of vertebrae, F. gymnauchen was most similar to T. obscurus (Hwang et al., 2018), and the number of vertebrae for the different species is as follows: F. gymnauchen was 26, L. grandis was 36 to 37 (Yun et al., 2020), T. obscurus was 25 to 27 (Hwang et al., 2018), C. laevis was 33 (Kim & Han, 1989), and L. guttatus was 36 to 37 (Kim et al., 1992).
Despite having a similar number of vertebrae, F. gymnauchen and T. obscurus (Hwang et al., 2018) have distinct external morphological differences in the broodstock, but these are difficult to distinguish during the larval period. F. gymnauchen and T. obscurus (Hwang et al., 2018) showed similar characteristics in terms of the anus location of hatched larvae, number of myotomes, and melanophore distribution during the morphological development of the larvae and juveniles. However, this study confirmed differences in the development of the vertebrae and urostyle bone.
During the larval and juvenile period of Gobiidae fish, many species have similar external morphological characteristics, making it difficult to identify species. Therefore, anatomical information from larvae and juvenile fish are important data for distinguishing similar species, and further research in this area is required.