INTRODUCTION
In general, the determination of animal sex is based on their morphological sex. Sex of bivalves can be categorized as either gonochoristic or hermaphroditic, whereas hermaphrodites can be described as either simultaneous or sequential (Coe, 1943; Heller, 1993; Yusa, 2007). Sequential hermaphrodites undergo sex changes during their life cycle, and their morphological sex is expressed differently according to the life cycle stage. The identification of sequential hermaphroditism requires direct evidence of sex change (Coe, 1943; Lee et al., 2013, 2014). However, since obtaining direct evidence requires considerable time and effort, an indirect method is used to analyze the change in sex ratio according to age or size within the same population (Galtsoff, 1937; Guo et al., 1998; Eversole, 2001; Park et al., 2012). The purpose of this study was to analyze the sex ratio of the mussel Mytilus coruscus, which is known to be gonochoristic (Wi et al., 2003), to thus describe its sex changes and sex.
MATERIALS AND METHODS
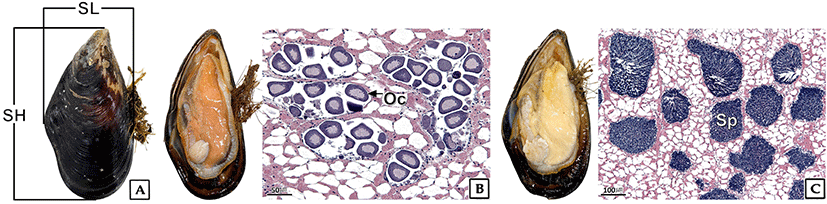
Mussels were collected from the subtidal region of Jumunjin on the eastern coast of Korea in July 2020 (Fig. 1). A total of 338 mussels of shell height (SH) 20.8–149.8 mm were used for sex ratio analysis. Sex was confirmed by observing anatomical and microscopic tissue specimens (Fig. 2). The sex ratio was recorded by dividing into classes of SH 10.0 mm each. The sex ratio (F:M) was expressed as the ratio of males (M) to females (F) and the female proportion (%) of the total, and statistical analysis was performed using the Chi-square (χ2) test.
RESULTS AND DISCUSSION
The overall sex ratio of mussels was approximately 1:0.7. However, the sex ratio according to the class of SH was different, where the male proportion was high in classes of SH 20.1–50.0 mm, but the female proportion was high in the SH 50.1–70.0 mm classes. The ratio of males was higher again in the SH 70.1–80.0 mm class, whereas the ratio of females was higher in the above SH 80.1 mm classes (Table 1). These results indicate that mussels are sequential hermaphrodites that periodically change sex (♂ → ♀ → ♂ → ♀).
Sex change normally occurs during the gonadal inactive stage in bivalves include Crassostrea virginica, C. gigas, Ruditapes philippinarum and Tegillarca granosa (Thompson et al., 1996; Park et al., 2012; Lee et al., 2013, 2014). However, sex change in Patinopecten yessoensis occurs during the gonadal active stages (Osanai, 1975). Genetic and environmental factors such as temperature affect the sex determination and change of sex ratio in bivalves (Yusa, 2007). It was confirmed in this study that the sex ratio and change according to shell size of M. coruscus. Therefore, more detailed research is needed on the season and factors involved in the sex changes of M. coruscus.