INTRODUCTION
Bisphenol A (BPA) is a chemical monomer used world-wide in the manufacture of polycarbonate plastics and epoxy resins and found in many products such as food and drink containers, toys, thermal paper, dental sealants, medical devices, flame retardant materials and so on (Michałowicz, 2014). As a result, humans have been constantly exposed to BPA through dietary and non-dietary sources. BPA and its metabolites are found in the majority of human body fluids including blood and urine. In analysis by the CDC BPA is present in 92.6% of human urine samples (Calafat et al., 2008). Moreover, BPA is also found in human ovarian follicular fluid, placental tissue, and fetal serum (Ikezuki et al., 2002; Schönfelder et al., 2002). The presence of BPA in maternal reproductive tissues and fluids indicates that BPA exposure can occur at developmental stages and thus, it has potential to affect subsequent generations through the germ line.
Several studies have examined the effects of embryonic BPA exposure on reproductive outcomes using rodent models. Perinatal exposure to BPA induced a decline in fertility and fecundity in the F1 generation of CD-1 mice (Cabaton et al., 2011). Further, in utero exposure to BPA disrupts ovarian development and estrous cycle in the F1 generation of FVB mice (Wang et al., 2014). In the same study, the BPA exposure also significantly reduced fertility in the F1 females (Wang et al., 2014). Moreover, in utero BPA exposure can compromise some female fertility phenotypes in the F2 and F3 generations suggesting that some effects may be transgenerational (Ziv-Gal et al., 2015).
In this report, the maternal effects of BPA on the fecundity in F1 and F2 generations were examined using Drosophila melanogaster. Drosophila melanogaster, due to its short lifespan and well-defined genetics, offers many advantages for the detection of mutagenic, morphological and developmental effect of chemical agents over generations. Exposure to environmentally relevant concentrations of BPA significantly decreased the fecundity of F1 and F2 female flies, showing maternal and transgenerational effects of BPA on Drosophila melanogaster reproductive function.
MATERIALS AND METHODS
In this study, the wild type w1118 fly was used. The flies were kept in a Drosophila culture room at 25℃ and relative humidity of 50%–60 % and in 12 h light, 12 h dark periods on a standard cornmeal Drosophila medium. Virgin female flies (2–3 day old) were exposed to 0, 0.1, 1, and 10 mg/L BPA in the standard medium. After 24 hours, the exposed 20 females and non-exposed 10 males of same age (3–4 day old) were crossed in the standard medium without BPA. 10 days later, F1 virgin females were collected and incubated in the standard medium. After 3 days, 20 F1 virgin females were crossed with 10 unexposed males of same age (3-day-old) in the grape juice agar plates. These plates were changed for every 24 h and the eggs were counted for a period of 3 days. After counting, the eggs were reared on the standard medium and F2 virgin females were collected. Twenty 3-day-old F2 virgin females were crossed with 10 unexposed males of same age and their eggs were counted in the same way as the eggs laid by the F1 females were counted. The results were expressed as percentages relative to controls (F1 or F2 females generated using F0 females exposed to 0 mg/L BPA). To compare control and test groups, we used a one-way ANOVA with Sidak correction. The statistical analysis of the results was carried out using the Prism program.
RESULTS AND DISCUSSION
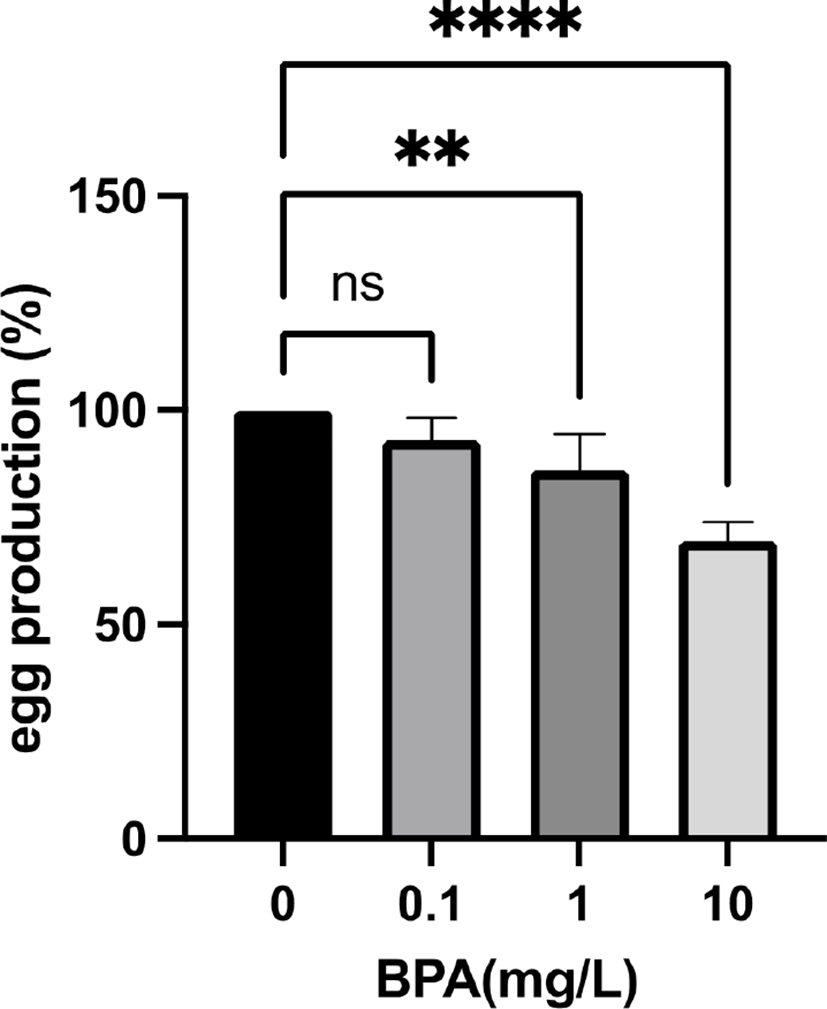
In previous studies, Vimal et al. reported that BPA exposed female flies have smaller ovaries with fewer mature oocytes and abnormal ovarian follicles (Vimal et al., 2019). The fecundity of these exposed females is also reduced and their eggs are defective in maternal transcripts and proteins (Vimal et al., 2019). The negative effect of BPA on female fertility was also observed in another Drosophila melanogaster study (Atli, 2013). To test the maternal effect of BPA exposure on fly reproductive function, we exposed Drosophila melanogaster females to environmetally relavant concentrations (0, 0.1, 1, and 10 mg/L) of BPA and crossed with non-exposed males on the standard fly food without BPA. The F1 generations were reared on the same fly food and their fecundity was checked (Fig. 1). Although 0.1 mg/L BPA had no effect on F1 fly fecundity, 1 mg/ L BPA significantly reduced the fecundity compared to controls. Moreover, 10 mg/L BPA further decreased the fecundity of F1 females showing that the effect of BPA on female fecundity in the F1 generation is dose dependent. These data demonstrated that maternal exposure to BPA decreases female reproductive function in Drosophila melanogaster.
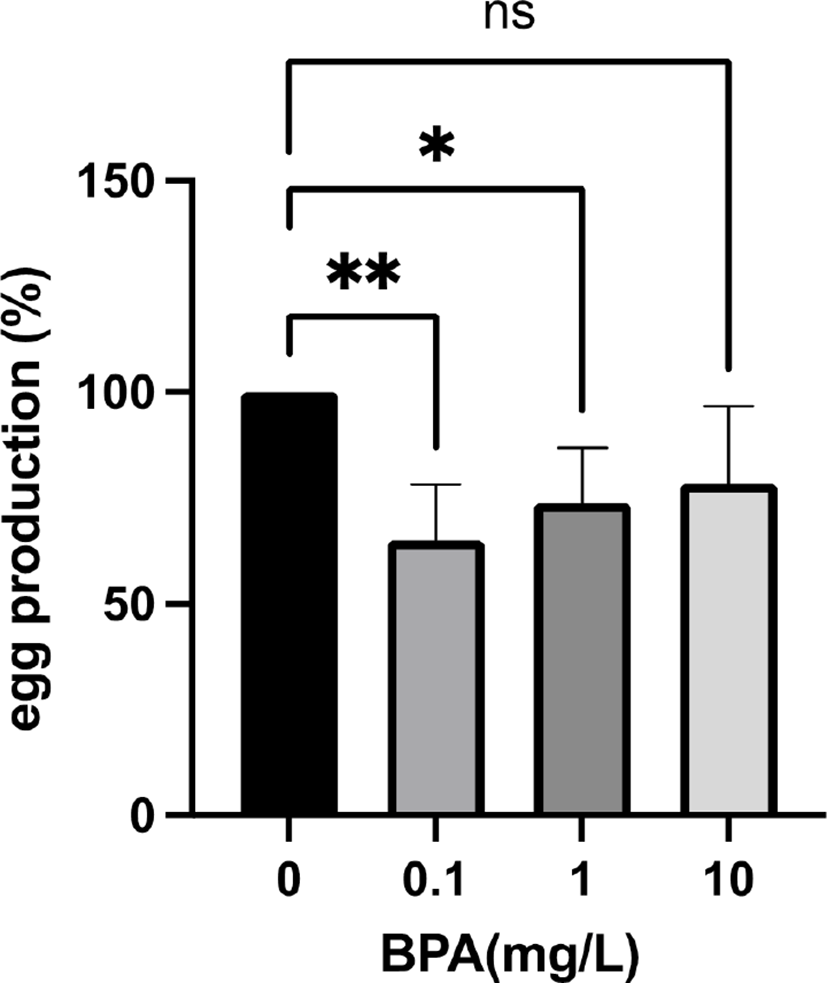
To test whether the effect of BPA on the fly fecundity is carried over next generation, we generated F2 females using the F1 females. Surprisingly, when F0 grandmothers were exposed to 0.1 mg/L and 1 mg/L BPA, the egg production of their F2 granddaughters was significantly decreased (Fig. 2). In contrast, 10 mg/L BPA exposure had no effect on the fecundity in the F2 generation (Fig. 2), showing that environmentally more relevant concentration (0.1 mg/L and 1 mg/L) of BPA can reduce the fly fecundity over generations. Interestingly, Ziv-Gal et al. reported that lower concentration of BPA can induce more profound decline in fertility of female mice over generations (Ziv-Gal et al., 2015). Moreover, Alworth and colleagues demonstrated that in utero exposure of CD-1 mice to diethylstilbestrol (DES), another endocrine disrupting chemical (EDC), caused opposite responses between low and high doses (Alworth et al., 2002). Under administration of estradiol, the low dose (0.1 μg/kg) of DES increased uterine weight, while the high dose (100 μg/kg) significantly reduced uterine weight compared to controls (Alworth et al., 2002). These data indicate that the high dose of BPA directly affects egg production in the F1 generation, while the lower dose of BPA reduces female reproductive function in the next generations by inducing long lasting changes such as epigenetic modifications. Therefore, the epigenetic alterations in BPA exposed female flies and their offspring across dose (from 0.1 to 10 mg/L) will be our next topic.
The transgenerational effects of EDCs on female reproduction in mice and rats have been reported by various research groups (Manikkam et al., 2013; Bruner-Tran et al., 2016; Zhou et al., 2017; Rattan et al., 2018). However, the rodent models have some limitations such as relatively long generation time and difficulties in genetic manipulation. As Drosophila melanogaster has many advantages such as short generation time (10–12 days) and powerful genetics, our fly model can be useful to study transgenerational effect of BPA and other EDCs to female fertility.
