INTRODUCTION
Di(2-ethylhexyl)phthalate (DEHP) is one of the highest production chemical and ubiquitously present in environment because of the widely usage to improve flexibility in polyvinylchloride (PVC) such as building materials and medical products (Shelby, 2006). It can release into the environment from the products and enter human body through direct contact and use the consumer products. As a result, the general population is continuously exposed to DEHP through ingestion, inhalation and dermal absorption (Shelby, 2006). Although DEHP has relatively short half-lives and rapidly excreted through urine or feces (Koch et al., 2004; Frederiksen et al., 2007), DEHP and their metabolites were detected in various biological samples, including urine (Blount et al., 2000; Koch et al., 2006; Park et al., 2010), in serum (Silva et al., 2005), and in breast milk (Mortensen et al., 2005).
While most studies have been focused on the reproductive toxicity of DEHP exposure (Akingbemi et al., 2001; Park et al., 2002; Lyche et al., 2009), the plausible association between DEHP and metabolic disorders, including obesity, diabetes mellitus, and hepatic lipid accumulation has been raised (Gayathri et al., 2004; Martinelli et al., 2006; An et al., 2021). In epidemiological studies, the association has also been reported between phthalates levels and the occurrence of metabolic disorders, including abdominal obesity and insulin resistance (Stahlhut et al., 2007), and nonalcoholic fatty liver disease (Yang et al., 2021). It seems that DEHP could interfere with not only reproduction system but also the endocrine system.
Thyroid hormones which have an important role in regulation of the growth, metabolism, and energy homeostasis (Mullur et al., 2014). Thus, the development of metabolic disorders after DEHP exposure might be linked with the alterations of thyroid hormones. In DEHP treated animals, the histological alteration of thyroid gland and the changes of thyroid hormone levels were observed (Price et al., 1988; Gayathri et al., 2004). In addition, urinary phthalate metabolites levels was associated with the alteration of thyroid hormones in adults (Meeker et al., 2007). It seems that even small changes in thyroid homeostasis may induce adverse effects. However, experimental studies have been used relatively higher dosages of DEHP than the environmental relevant levels. As a result, some responses and effects of DEHP at environmental relevant levels on thyroid hormones still need to explain.
Therefore, this study focused on the influences that short term exposure to DEHP may contribute to the alteration of thyroid hormones in adolescent male rats.
MATERIALS AND METHODS
Four week old Sprague-Dawley (SD) rats were used in this study. Specific pathogen-free (SPF) SD rats were purchased from the Charles River breeding laboratory (Wilmington, MA, USA). The rats were housed in an animal room controlled at 23±3°C with a relative humidity of 55±15% under a light/dark cycle of 12 h (light from 20:00 to 08:00 hours). All animals were provided with free access to water and an appropriate diet. After acclimatization to the light/dark cycle for 1 week, the experiment was started. The rats were randomly assigned to five groups (n=5/group), including control (vehicle) and DEHP 0.75, 7.5, 15, and 150 mg/kg/day treated groups. Dosing solution was prepared by thoroughly mixing DEHP in corn oil at the proper concentration. The rats were treated with once daily by oral gavage 1 week before being sacrificed. Dosing volumes were 10 mL/kg. The dosing solution was prepared by thoroughly mixing DEHP in corn oil at the proper concentration. This study was conducted in accordance with the Good Laboratory Practice (GLP) guidelines for Animal Experiments defined by the Korea Testing and Research Institute (TBH-1252, 2009/12).
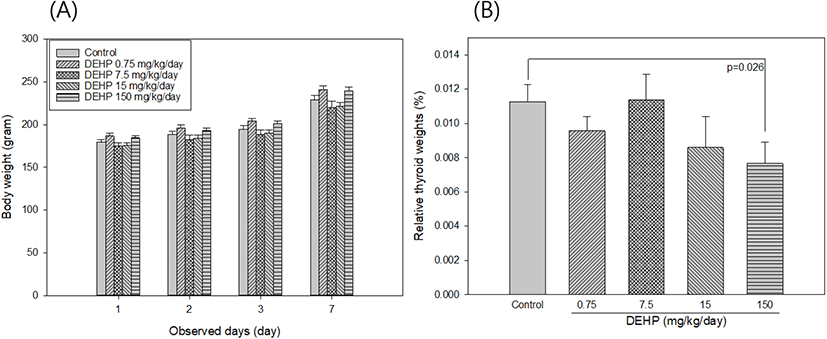
SD rats had their body weight measured 1, 3, and 7 days after treatment. The weight of the thyroid was measured after necropsy and then relative thyroid weight was calculated by thyroid weight (g) / body weight (g) × 100.
Blood samples were obtained from the heart after sacrifice. The samples were kept at 5°C until centrifugation at 1000×g. Serum was distributed into sterile Cryo Vials (Greiner Labortechnik, Frickenhausen, Germany) in volumes of 500 μL and immediately frozen at −80°C until analysis. The levels of plasma thyroid hormones, including triiodothyronine (T3), thyroxine (T4), and thyroid stimulating hormone (TSH) were measured by enzyme-linked immunosorbent assay according to the manufacturer’s protocol (Endocrine Technology, Old Bridge, NJ, USA). The absorbance was measured 450 nm using a plate reader (Tecan Sunrise TW, Salzburg, Austria).
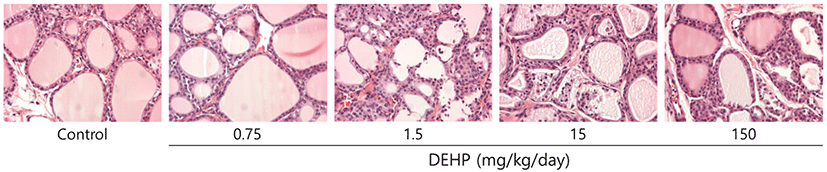
Thyroid was preserved and fixed in 10% neutral solution buffered with formalin, after which it was washed several times with ethanol (70%) before being embedded in paraffin. The embedded tissue was then sectioned at a thickness of 5 μm and then stained with Periodic Acid Schiff (PAS) and hematoxylin-eosin (H&E). Stained slides were analyzed by light microscopy (Olympus, Tokyo, Japan).
RESULTS AND DISCUSSION
DEHP is widely used in plastic products to increase flexibility, thus, human can be exposed to DEHP through ingestion, inhalation, and absorption. It has been suggested that DEHP might associate with the development of metabolic disorders. Because thyroid hormones are important to regulate the glucose and lipid metabolism (Mullur et al., 2014), the alteration of circulating thyroid hormones due to DEHP might induce metabolic disorders through interfering with the binding of their receptors or transport proteins. Therefore, this study was conducted to identify thyroid hormones altered by short-term exposure to DEHP.
Thyroid hormones, T3 and T4 are released from thyroid gland, and are regulated by TSH in pituitary gland. When thyroid are not working properly, the circulating levels of thyroid hormones are altered and resulted in numerous disease occurrence, including cardiovascular disease, osteoporosis, and neurological function (Boelaert & Franklyn, 2005). Age, gender, BMI, family history of thyroid disease, smoking, estrogen, and pregnancy are known as risk factors of thyroid dysfunction. Recently, there is growing the considerable attention of DEHP on the development of poor thyroid function. The inverse association of urinary levels of DEHP metabolites with free serum T4 and T3 in men (Meeker et al., 2007) and with total T4 and free T4 in pregnant women (Huang et al., 2007) was previously reported. In animal studies, decreased plasma T4 levels were observed in rats fed with DEHP compared to the control (Howarth et al., 2001). In this study, after 1 week exposure to DEHP, there were no significant differences in body weights between control and DEHP treated groups (Fig. 1A). Relative thyroid weights significantly decreased in DEHP 150 mg/kg/day treated SD rats compared to the control rats (p=0.026) (Fig. 1B). In addition, exposure to DEHP 0.75 mg/kg/day and 150 mg/kg/day were significantly reduced serum T4 levels compared to the control group (p=0.015 and p=0.015, respectively [Table 1]). While serum TSH and T3 levels in serum were not detected the significant changes between control and DEHP treated groups.
DEHP, di(2-ethylhexyl)phthalate; TSH, thyroid stimulating hormone.
In addition, previous studies have reported the histological alterations including increase of number and size of lysosomes, and hyperplasia after DEHP exposure (Price et al., 1988; Gayathri et al., 2004); while this study was not observed. It might be due to the relatively short-term of exposure (1 week) and low dose of DEHP (0.75, 7.5, 15, and 150 mg/kg/day). The treated dosages in this study were determined by considering the exposure levels of DEHP in general population (approximately 5-100 ug/kg of body weight) (Tickner et al., 2001) and in patients receiving haemodialysis and blood transfusion (up to 4 mg/kg body weight/day) (Calafat & McKee, 2006). Also, the highest dosage (150 mg/kg/day) of DEHP can reflect the obvious thyroid toxicity. Despite the histological changes resulted from DEHP exposure were not detected in this study (Fig. 2), short-term exposure of DEHP disrupt thyroid hormone homeostasis.
The binding of DEHP to thyroid hormone receptors is suggested the underlying mechanism. The increase of iodide uptake after DEHP 10–4 and 10–3 M exposure were observed in FRTL-5 rat thyroid follicular cells (Wenzel et al., 2005). It might be due to the stimulation of sodium/iodide symporter (NIS) by DEHP in PC Cl3 rat thyroid cell line (Breous et al., 2005). However, adolescent male rats treated with DEHP 250, 500, and 750 mg/kg/day for 30 days were showed the decrease of thyroid hormone biosynthesis and NIS levels, and observed the follicular epithelial cell hypertrophy and hyperplasia (Liu et al., 2015). They suggested that the up-regulation of hepatic enzymes expression might reduce thyroid hormones. Thus, in order to evaluate the thyroid disrupting effects of DEHP, it is needed to consider the thyroid hormone levels, thyroid hormone synthesis-related proteins, deiodinases, receptors, and hepatic enzymes.
Consequently, short-term exposure to DEHP might lead the poor thyroid function at doses corresponding to transfusion of blood in a recipient. Further studies are needed to determine the long term exposure to DEHP.
