INTRODUCTION
Ascidians, also known as tunicates or sea squirts, are classified as chordates along with vertebrates because their tadpole-shaped larvae possess a notochord in the tail and a dorsal nerve cord (Kowalevsky, 1866; Katz, 1983; Nishida 2005; Lemaire, 2009). Ascidians are mainly consumed in Asia, Chile and the Mediterranean, where product is sourced from both the wild and, in the case of the Halocynthia and Styela species in high demand, from cultured populations (Nguyen et al., 2007; Lambert et al., 2016). The ascidian H. roretzi (sea pineapple) is not only one of the model animals in the field of developmental biology but also one of the major aquaculture varieties in Korea and Japan (Shenkar & Swalla, 2011). However, the farmed fishermen are experiencing a lot of difficulties because of the mass death of H. roretzi due to increase in water temperature, changes in the environment, and outbreak of the disease such as soft tunic syndrome (Kumagai et al., 2011; Kim et al., 2014; Shin et al., 2014).
The ascidian H. aurantium (sea peach) is a marine invertebrate belonging to the same genus of chordates with H. roretzi. It has been reported that H. aurantium inhabits the northern part of the East Sea, the Sea of Okhotsk, the Bering Sea, and the Gulf of Alaska in the Pacific Ocean (Van Name, 1945). In Korea, it is generally distributed up to the coast of Sokcho, and there is a report that it was also collected from the coast of Samcheok, Gangwon-do (Rho & Lee, 1991). Its usual range in depth is from about 10 to 100 m, but it is estimated that it mainly inhabits at a depth of 10 to 40 m on the coast of Gangwon-do.
H. aurantium is a strong candidate, as a new cultivated variety of ascidian species. H. aurantium and H. roretzi resemble each other not only in taste but also in many characteristics of food product. They are presumed to be similar in biological characteristics. However, the reproduction of H. aurantium is not well known despite some studies on the physiology, the early development, and the mitochondrial genome (Inazawa et al., 1998; Kim, 2020; Kim et al., 2021). In this study, we investigated gonadal changes during the annual reproductive cycle of the ascidian H. aurantium.
MATERIALS AND METHODS
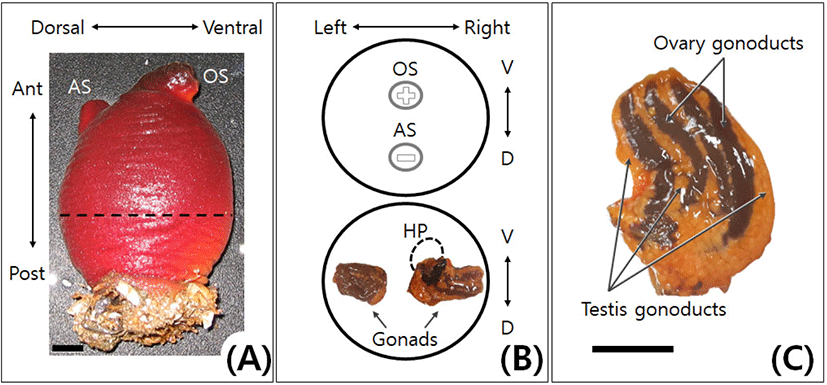
Adults of ascidians, H. aurantium (Pallas) and H. roretzi (Drasche), were collected in the sea near Sokcho, Gangwon-do, Korea from July 2015 to October 2016. To calculate the gonadosomatic index (GSI: gonad weight/body weight×100) of H. aurantium, the weight of the body and the gonad was measured using 10 individuals every month. The average size of H. aurantium and H. roretzi adults used in the experiment was 230 g and 250 g, respectively. The gonads were obtained by incision on the dorsal side (Atrial siphon side) of the Adults (Fig. 1).
RESULTS AND DISCUSSION
In adult of H. aurantium, the attached side is the posterior of the body. On the upper, the anterior side has an incurrent oral (branchial) siphon and an outcurrent atrial siphon (Fig. 1A). Oral siphon is located on the ventral side. When siphons are closed, oral siphon is cross-shaped, whereas atrial siphon is rod-shaped (Fig. 1B). Adult of the ascidian H. aurantium is a hermaphrodite like that of H. roretzi. The bisexual gonad of H. aurantium was a type of the ovotestes in which testis and ovary coexist (Fig. 1C). In H. aurantium, the right gonad was longer and slightly larger than the left gonad throughout the year (Fig. 1B, lower diagram). The hepatopancreas was attached only to the right gonad. In each gonad of H. aurantium, the number of the testis gonoducts was slightly higher than that of the ovary gonoducts (Table 1). Moreover, the number of the testis and ovary gonoducts in each gonad of H. aurantium was about half that of the H. roretzi. The gonoducts of testis and ovary were alternately located in the bisexual gonad, and the testis gonoducts were mainly located on both edges of the gonad (Fig. 1C). When the gonads were reduced, the testis gonoducts atrophied earlier than the ovary gonoducts (data not shown). These features were similarly observed in H. roretzi (Kim et al., 2001).
To find out the spawning period of H. aurantium, we investigated development of the gonads during the annual reproductive cycle. Development of the gonads was determined using GSI. GSI of H. aurantium showed the highest level in September 2015, and then gradually decreased to the lowest level in February of the following year (Fig. 2). The GSI has gradually increased since March, and reached the highest level again in September. The average GSI in September, the highest level, was about 3.8 times higher than that in February, the lowest level. It seems to be that the gonads of H. aurantium contracted from winter to next summer seasons. The gonads decreased to the smallest size around February, and then started to increase in March. The gonads were most developed in September in both 2015 and 2016. Therefore, it is likely that the spawning period of H. aurantium begins around September.
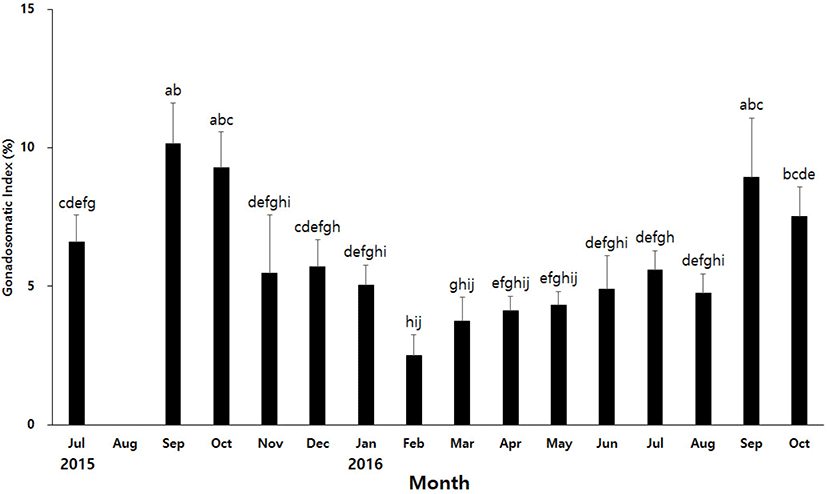
As in the case of many marine animals, the spawning of ascidians is known to be affected by the photoperiod and water temperature, so there is a difference depending on the habitat (Bates, 2011; Shenkar & Swalla, 2011). In the East Sea of Gangwon-do, the spawning period of H. roretzi has been reported between December and February (Kim et al., 2001). It was reported that the spawning of H. hilgendorfi ritteri living in the sea near Jeju Island occurs from November to January (Choi et al., 2004). These two species belonging to the Halocynthia genus are a type of winter breeder. In contrast, H. aurantium appears to spawn in autumn. There is a possibility that the spawning periods of H. aurantium is likely to vary slightly depending on the depth of the habitat. The water temperature at a depth of 10 m and 30 m differs by more than 10 degrees depending on the season. Thus, it is important to know the habitat depth of H. aurantium used in the experiment, but it is very difficult to collect only the individuals of a certain depth in the current situation where aquaculture is not possible. There can be a difference in the spawning periods of H. aurantium living a depth of 10 m and a depth of 30 m or less, but the difference will not be very large. Therefore, these results will be useful for future studies on the development and reproduction of H. aurantium.
