INTRODUCTION
Human pluripotent stem cells (hPSCs), including both human embryonic stem cells (hESCs) and induced PSCs (hiPSCs), have an unlimited capacity to differentiate into a variety of biologically relevant mature cell types, which offer unique in vitro models for predicting potential cytotoxicity of environmental toxins (Lee & Son, 2021). In fact, cytotoxicity of heavy metals, nanoparticles, endocrine disruptors, water-borne toxins have been evaluated in hPSC-derived immature and mature cell types including cardiomyocyte, endothelial cells, neural progenitor cells, and hepatocytes (Rajamani et al., 2017; Tang et al., 2017; Hong et al., 2019; Vanova et al., 2019). All these reports suggest that hPSC derivatives have the potential to be used in cytotoxicity assessment of various harmful materials, and could be more biologically relevant alternatives for the replacement of human primary cells and cell lines.
Recently, several research groups have generated functional alveolar epithelial cells (AECs) from hPSCs and showed their promising applications for drug efficacy testing, disease modeling and infectious study to treat acute and chronic respiratory diseases (Huang et al., 2020; Heo & Hong, 2021; Kim et al., 2021; Suezawa et al., 2021). We have previously investigated the cytotoxicity of cadmium (Cd) in hPSC-derived-AECs and human primary AECs (hpAECs) and found similarities in the cellular responses to Cd exposure (Heo et al., 2019). We have also demonstrated that hPSC-derived three-dimensional (3D) alveolar organoids system could be a robust in vitro platform for assessing the adverse effects of diesel particulate matter (dPM) (Kim et al., 2020). However, hpAECs derived from different donors can exhibit distinct responses depending on age and genetic background. Moreover, the characteristics of hpAECs may change due to in vitro culture for long-term maintenance. These studies allow us to extend the utility of hPSC-AECs for in vitro pulmotoxicity prediction of various environmental toxins. Therefore, in the present study, we employed hPSC-AECs to evaluate their responses to exposure of various concentrations of dPM, cigarette smoke extract (CSE) and nicotine in terms of cell death, inflammation, and oxidative stress.
MATERIALS AND METHODS
hpAECs (ScienCell, Carlsbad, CA, USA) were cultured in AEC medium (ScienCell) supplemented 10% fetal bovine serum (HyClone Laboratories, Logan, UT, USA) on dish coated with poly-L-lysine (Sigma-Aldrich, St. Louis, MO, USA). Human PSCs (CHA15) were kindly provided by CHA University (Pocheon, Korea) and maintained as previously described (Kim et al., 2021). Briefly, the cells were cultured using E8 medium (STEMCELL Technologies, Vancouver, BC, Canada) on dishes coated with Matrigel (BD Bioscience, Franklin Lakes, NJ, USA). Cells were subcultured at 70%–80% confluency and passaged every 4–5 days by mechanical dissociation. All cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
Multistep AEC differentiation was performed as previously described (Heo & Hong, 2021). Briefly, undifferentiated hPSCs were dissociated and then plated in dishes coated with Matrigel. After an overnight incubation, AEC differentiation was initiated with exposure to stepwise induction medium and assessed by measuring the frequencies of alveolar epithelial progenitors (AEPs) and AEC-specific markers on day 25 post-initiation using flow cytometry.
The dPM (Diesel Particulate Matter, NIST, SRM® 1650b) was purchased from Merck Millipore (Merck Millipore, Darmstadt, Germany). A stock and working solutions of dPM were prepared as previously described (Kim et al., 2020). hpAECs and hPSC-AECs were treated with dPM at concentrations of 0, 50, 100, and 200 g/mL, respectively, for 48 hrs. CSE was prepared as previously described (Lee et al., 2018). Briefly, using one 3R4F cigarette (The Tobacco Research Institute, University of Kentucky, Lexington, KY, USA), cigarette smoke was bubbled through 10 mL of serum-free DMEM/F12 supplemented with 1% penicillin-streptomycin. Then, the solution containing smoke was filtered through a 0.22 μm filter to remove large particles and was regarded as a 10% CSE solution. The cells were stimulated 1, 10, and 100 μM S(−)-nicotine (N-008, Merck Millipore) for 48 hrs after plating.
hpAECs (1×103 cells/well) were seeded in a 96-well plate and incubated overnight to attach. Cells were grown to 90% confluence and then were treated with or without various concentrations of dPM, CSE and Nicotine for 48 hrs to determine subtoxic doses in vitro. The cells were replaced with fresh medium followed by further incubation with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Abcam, Cambridge, UK) for 4 hrs. MTT solution was removed followed by measuring the absorbance at 490 nm using spectrophotometer.
Total RNA was extracted from hPSC-AECs using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA was reverse-transcribed into cDNA using TOPscript™ RT DryMIX kit (Enzynomics, Daejeon, Korea). Quantification of mRNA expression for candidate genes was performed by qPCR using ABI Step One Plus System Instrument (Applied Biosystems, Waltham, MA, USA). Relative expression level of mRNAs was calculated using 2-ΔΔCT method following normalization to GAPDH. The primer sequences were provided in Table 1. The qPCR thermal cycle condition used was 30s denaturation at 95°C, 45s annealing at 58°C–62°C, and extension for 45s at 72°C.
hPSC-AECs were incubated with collagenase IV (Sigma-Aldrich) for 2 hrs, followed by treatment with cell dissociation buffer (Gibco, Grand Island, NY, USA) for 30 min at 37°C. Cells were passed through a 70 μm cell strainer and incubated with following primary antibodies for 1 hr at 4°C: NKX2.1 (Abcam), carboxypeptidase M (CPM), epithelial cell adhesion molecule (EPCAM, Santa Cruz, Dallas, TX, USA), Surfactant protein B (SFTPB, EMD Millipore, Burlington, MA, USA), SFTPC (Abcam), T1α and aquaporin 5 (AQP5). Dead cells were excluded by 7-aminoactinomycin D (BD Pharmingen, San Diego, CA, USA) staining. Frequencies of AEC markers were measured using a FACSCantoTM II flow cytometer (BD Bioscience), and acquired data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
RESULTS AND DISCUSSION
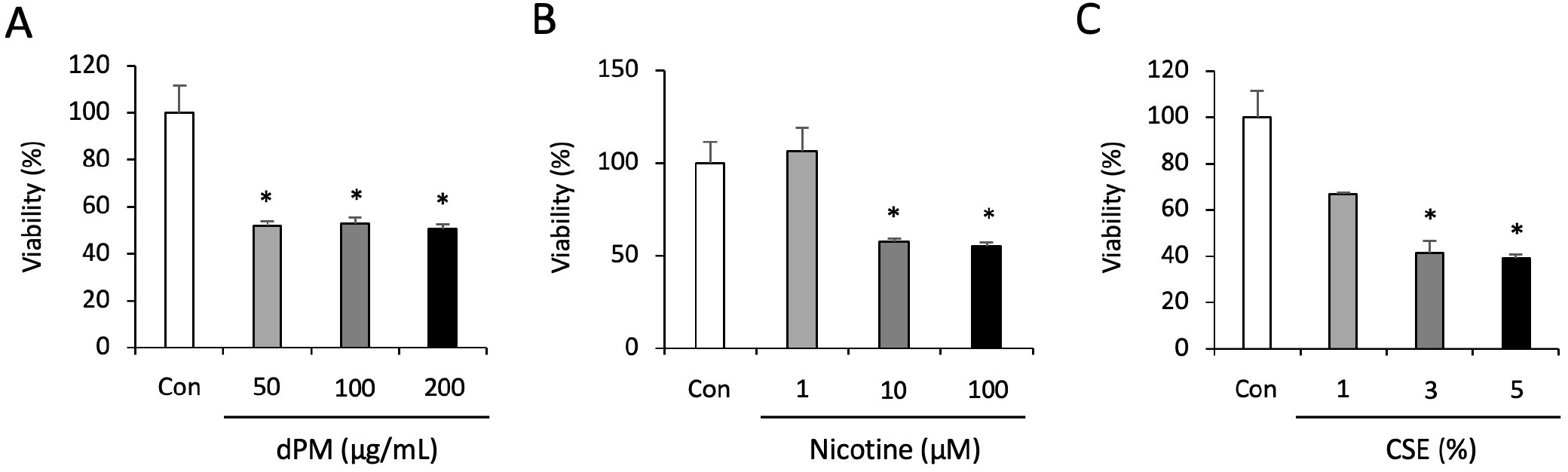
To evaluate the toxic effects of dPM, nicotine and CSE on hpAECs, the viability of hpAECs exposed to various concentrations for 48 hrs was measured using MTT assay. A significant toxic effect of dPM on viability was observed in cells treated with 50–200 μg/mL (Fig. 1A). A significant toxic effect of nicotine on viability was observed in cells with 10 and 100 μM, but not in cells treated with low concentration (1 μM) (Fig. 1B). Similarly, higher concentrations (3% and 5%) of CSE significantly affected the viability of cells, an effect which was not observed with 1% CSE (Fig. 1C). On the basis of the results of MTS assay, we further investigated if these toxic materials influence the transcription of cell death, inflammation and oxidative stress gene-related genes in hPSC-derived AECs.
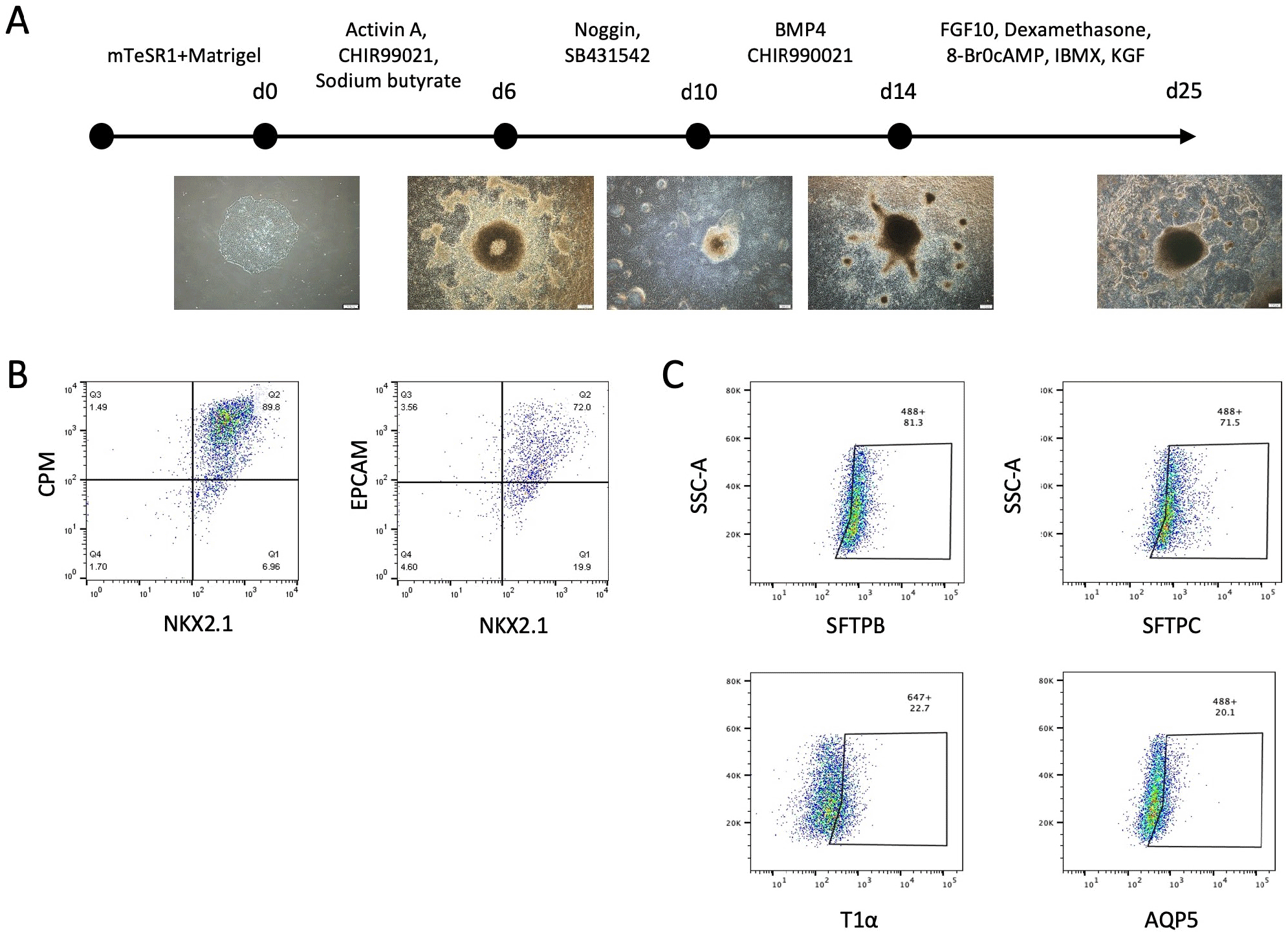
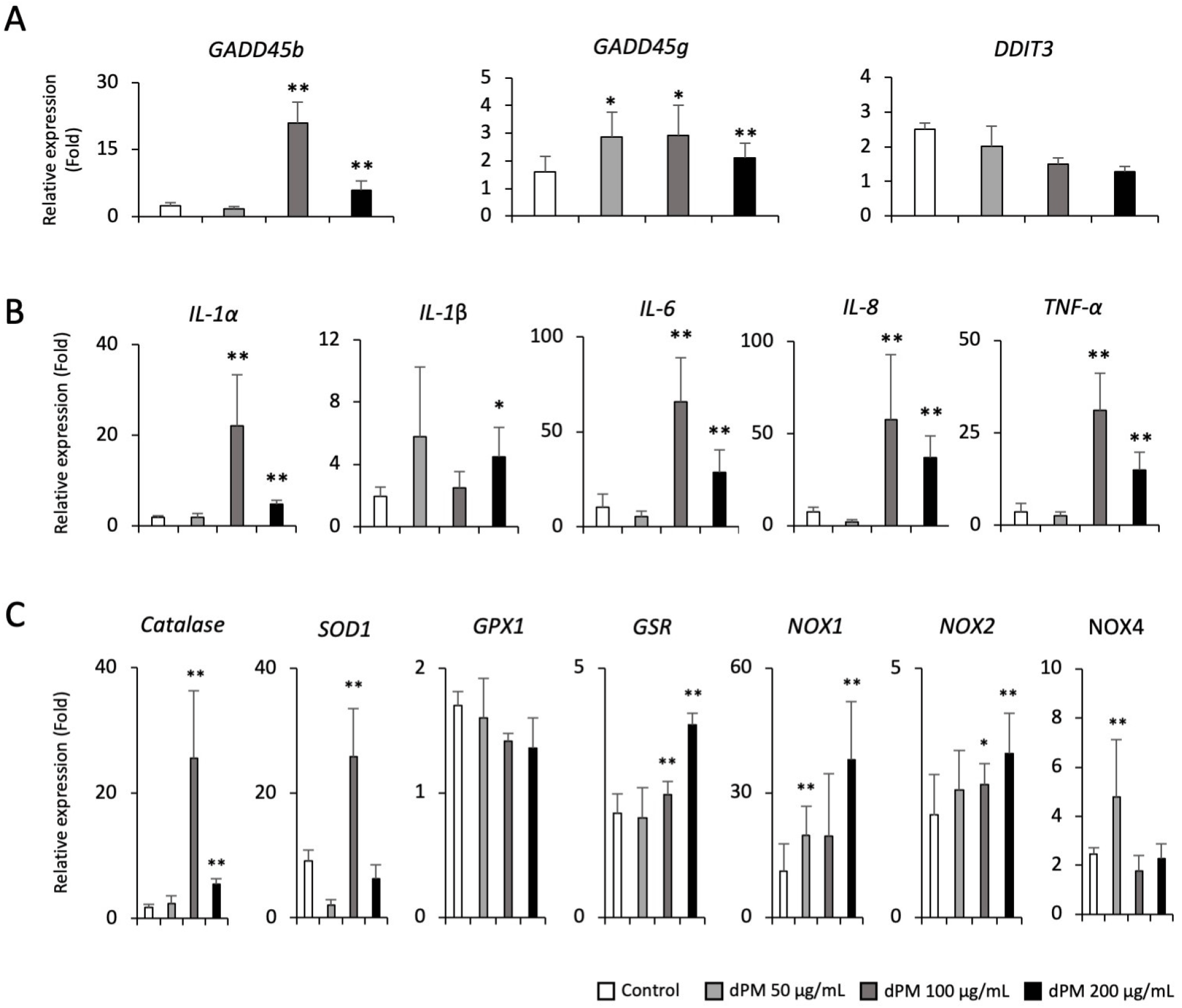
We have previously reported efficient and stepwise induction protocol for the generation of AEPs and functional AECs from hPSCs (Fig. 2A). Flow cytometry analysis showed that hPSC-AEPs and –AECs express their specific markers (NKX2.1 and CPM for AEPs; SFTPB and SFTPC for type II AECs; T1α and AQP5 for type I AECs) (Fig. 2B and C). Using hPSC-AECs, we first evaluated the cytotoxicity of dPM with various concentrations (50, 100, and 200 μg/mL). We found that high concentration (100 and 200 μg/mL) of dPM upregulated the transcription of the growth arrest and DNA damage-induced 45-beta (GADD45b) and –gamma (GADD45g) as well as pro-inflammatory cytokines and mediators such as interleukin (IL)-1α, IL-1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α (Fig. 3A and B). In addition, ROS-related genes including Catalase, superoxide dismutase 1 (SOD1), glutathione-disulfide reductase (GSR), and NADPH oxidase 1/2/4 (NOX1/2/4) were also significantly were increased in dPM-treated cells compared to the control (Fig. 3C). These results suggest that dPM treatment induces cellular stress by promoting apoptosis, inflammation and oxidative stress, which may impair alveolar homeostasis and lead to chronic respiratory disease such as pulmonary fibrosis and chronic obstructive pulmonary disease.
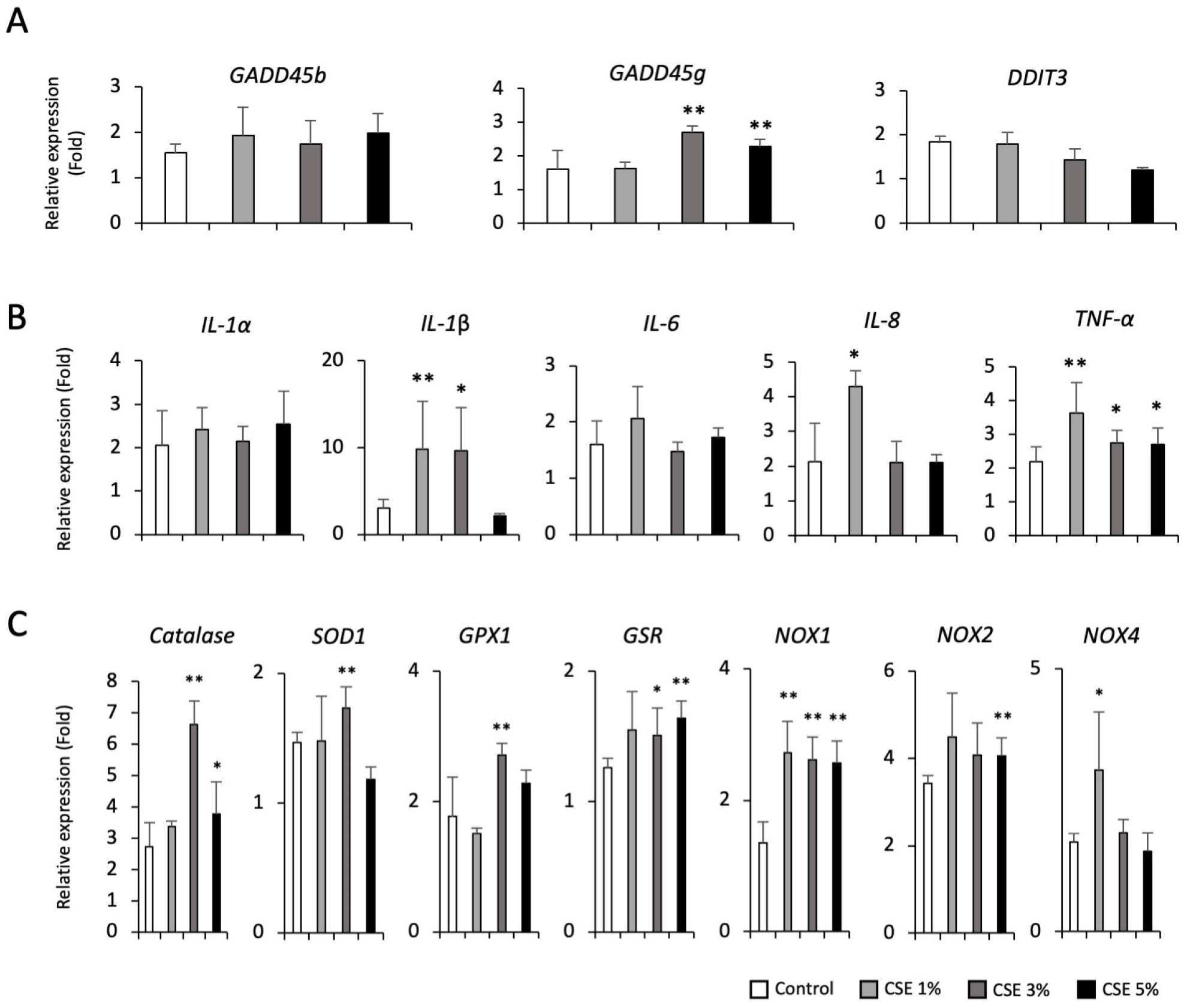
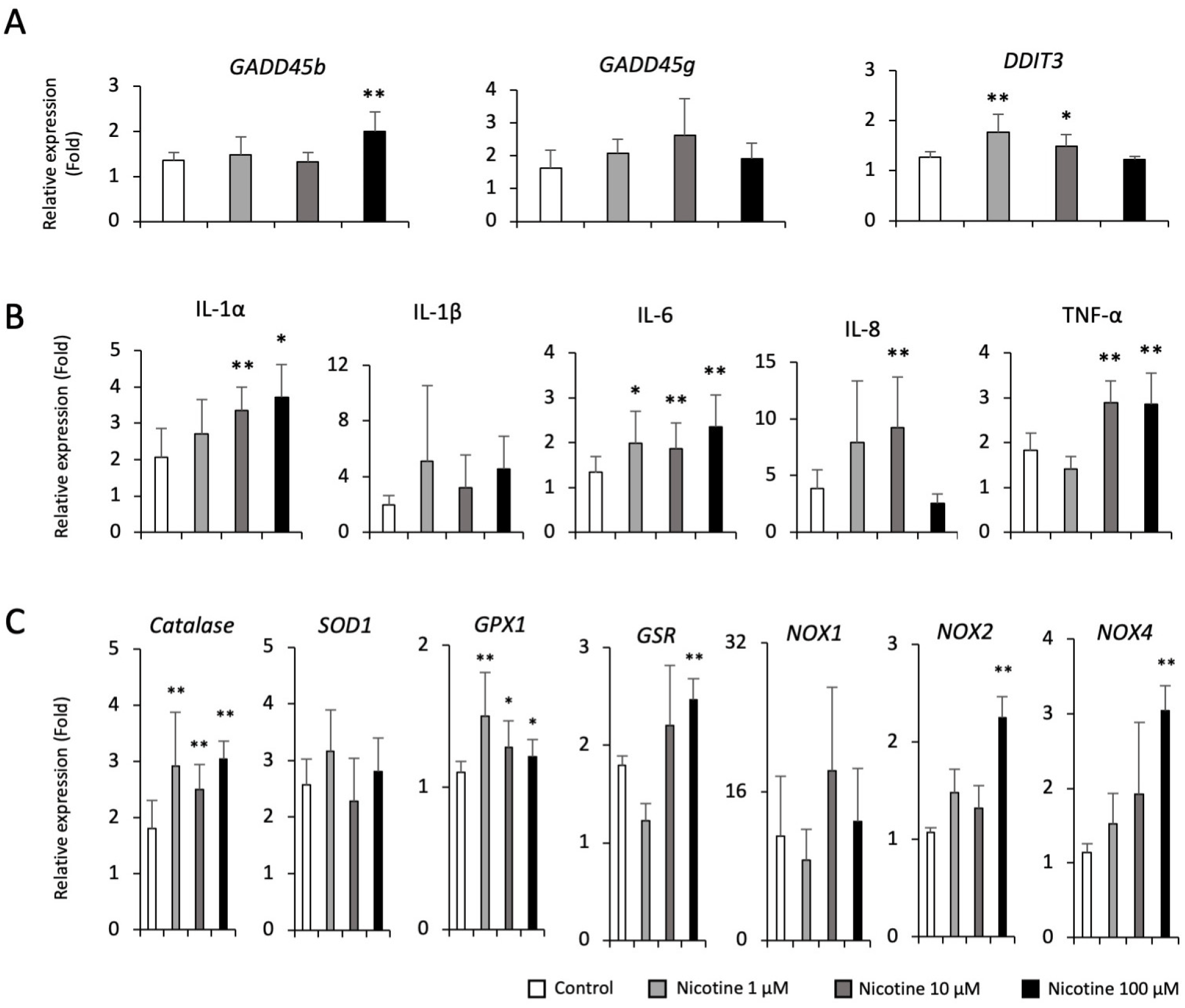
We next investigated the effect of nicotine exposure on apoptosis, inflammation and oxidative stress in hPSC-AECs. We also found the upregulation of GADD45b gene in high dose (100 μM) of nicotine (Fig. 4A), but not as high as in high concentrations of dPM (100 and 200 μg/mL). However, transcript levels of pro-inflammatory cytokines such as IL-1α, IL-6, IL-8, and TNF-α were significantly upregulated in a dose-dependent manner (Fig. 4B). In addition, Catalase, glutathione peroxidase 1 (GPX1), GSR, NOX2, and NOX4 transcripts were significantly increased in nicotine-treated cells compared to the control (Fig. 3C). These findings suggest that inhalation exposure to nicotine may related to the early development of lung diseases by promoting inflammatory responses and oxidative stress. Lastly, we examined the alterations in apoptosis, inflammation and oxidative stress-related genes in CSE-treated hPSC-AECs. Similarly, we found the upregulation of GADD45g gene in high dose (3% and 5%) of CSE, but not as high as in high concentrations of dPM (100 and 200 μg/mL) (Fig. 5A). Transcript levels of IL-1β, IL-8, and TNF-α were significantly upregulated in CSE-treated cells (Fig. 5B), but not as much as high concentrations of dPM (100 and 200 μg/mL). Furthermore, most of oxidative stress-related genes were also significantly upregulated in CSE (3% and 5%)-treated cells (Fig. 5C).
We have previously evaluated the developmental toxicity of dPM (50 and 100 μg/mL) during AEC differentiation and found a marked reduction of AEC specific genes (NXK2.1, Aquaporin 5 and T1α) and induction of fibrosis (COL1A1, α-SMA, and VIMENTIN)-and epithelial to mesenchyme (EMT) (SLUG, SNAIL1, TWIST and CTNNB1)-related genes (Kim et al., 2020). These findings suggest that dPM-induced genetic alterations in EMT and fibrosis might be involved in disturbing hPSC differentiation towards AECs. In this study, we further revealed the upregulation of apoptosis, pro-inflammation and ROS-related transcripts in hPSC-AECs treated with dPM.
Taken together, our results suggest that chronic respiratory diseases, lung development and irreversible deficits in lung function affected by dPM exposure may be initiated by chronic inflammatory response and oxidative stress. Although hPSC-AECs offer a robust in vitro tool to assess pulmotoxicity of various air pollutants and harmful chemicals, the 2D AEC cultures remain incomplete as they lack of key components of alveolar tissues, including immune cells and vessels. Moreover, recent progress in 3D organoid system has gained great attention for the replacement of 2D culture system (Lee et al., 2021; Heo et al., 2022; Kim et al., 2022; Kook et al., 2022; Kwon et al., 2022). Thus, development of 3D alveolar organoid system with integration of key missing components will facilitate a significant advancement in the in vitro toxicity screening platform.