INTRODUCTION
Mammalian hypothalamic-pituitary axis (HP axis) plays a critical and integrative role in the endocrine system control to maintain homeostasis (Graceli et al., 2020). In addition to the hormones that make up the HP axis, recent studies strongly suggest that endocrine disrupting chemicals (EDCs) such as bisphenol A (BPA), some phthalates and polychlorinated biphenyls (PCBs), is associated with HP axis abnormalities, exerting somewhat toxicological effects (Diamanti-Kandarakis et al., 2009; Zoeller et al., 2012). For example, BPA increased the expression levels of Gnrh1, Kiss1 and FSH mRNA after gestational and lactational exposure at doses higher than the sublingual dose (12, 25, and 50 mg/kg/d) in both male and female rodent pups, in a dose-dependent manner (Xi et al., 2011). While the hypothalamic-pituitary-gonadal axis is crucial for regulating reproduction, research on the hypothalamus within this axis has historically lagged behind studies of the pituitary and gonads (Acevedo-Rodriguez et al., 2018). The causes of the lag may include difficulties in measuring hypothalamic neurohormones and/or neurotransmitters, difficulties in obtaining tissues with clear boundaries, and the absence of suitable cell or organ culture methods. Previously, we developed a short-term incubation method of rat adrenal and demonstrated that in vitro nonylphenol (NP) exposure could induce changes in secretions of catecholamine and adrenocortical steroids (Kim et al., 2023). NP is a member of alkylphenols and is known to an EDC with weak estrogenic activity (Galązka & Jankiewicz, 2022), so this method could be quite suitable for evaluating endocrine disrupting activities rapidly and effectively. In addition, the adrenal medulla can be described as a modified sympathetic ganglion (Guérineau et al., 2020), hypothalamus which is known to release catecholamines may respond to EDCs exposure (Kaehler et al., 1999). Therefore, we considered it could be appropriate to use our incubation system to investigate the effects of EDCs on the release of neuroendocrine hormones from hypothalamus. Consequently, to extend the utility of the incubation method further, we tested the effects of NP for the first time on changes in hormonal secretion in the short-term incubated rat hypothalamus. Since the most important neuroendocrine peptides in the regulation of reproductive phenomena of the H-P axis are GnRH and its upstream regulator, kisspeptin, these two peptides were measured in this study (Gore, 2010; Ruohonen et al., 2020).
MATERIALS AND METHODS
Sprague-Dawley rats were provided by DBL (Eumseong, Korea) and reared in Sangmyung University animal facility under photoperiods of 12 h light/dark with lights on at 7 AM and constant temperature of 21°C–23°C. Food and tap water were supplied ad libitum. The animal protocols were approved by the Animal Care and Use Committees at Sangmyung University (2018-03; approval number R-1801-1). All the animals received human care in accordance with the guides for animal experiments of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
Short-term organ incubation of rat hypothalamus was based on previous report (Rettori et al., 1997; Kim et al., 2023) with minor modification. Hypothalami of 8 weeks-old female (in proestrus) and male rats were collected after decapitation; the mediobasal hypothalamus (MBH) was dissected by making a transverse cut just behind the optic chasm, extending dorsally 1.0 mm. A horizontal cut extended from this point caudally to just behind the pituitary stalk, where another frontal cut was made. Bilateral longitudinal cuts were made 1 mm lateral to the midline. The hypothalami were pre-incubated with a Krebs-Ringer Bicarbonate (KRB; Sigma-Aldrich, St. Louis, MO, USA) medium for 1 hour at 37°C in incubator (BINDER, Tuttlingen, Germany) aerated with 95% air and 5% CO2. Then the tissues were shifted in fresh media and treated with NP (100 pM, 1 nM, 10 nM, 100 nM or 1 μM) for 1 hour at 37°C. After incubation, the media were centrifuged (10,000×g for 30 min at 4°C, Hanil, Gimpo, Korea) and the supernatants were stored at –80°C until analysis.
Measurements of catecholamines, including norepinephrine (NE) and epinephrine, were conducted with reverse-phase high-performance liquid chromatography with electrochemical detection (RP-HPLC-ECD) using methods modified from Wood & Hall (2000). The concentration range of NP treatment was determined through our preliminary experiments to reflect realistic exposure in daily life. The samples were centrifuged at 10,000×g for 10 min at 4°C to remove precipitated proteins. The supernatants were diluted by mobile phase buffer [0.01 N Formic acid (Shinyo Pure Chemicals, Osaka, Japan), 0.05 mM 1-Octanesulfonic acid sodium salt (Sigma-Aldrich), 10% Methanol (Honeywell, Morristown, NJ, USA)] before determining catecholamine content. The mobile phases were prepared as needed and degassed by sonicator (KODO Technical Research, Hwaseong, Korea) prior to use. Standards and samples were analyzed at room temperature using 5 μM Waters Xbridge C18 (150×4.6 mm I.D.) column (Waters, Milford, MA, USA). Injection volumes of samples and standard were 10 μL (2707 Auto sampler; Waters). The flowrate was 1 mL/minute over 20 minutes with an isocratic elution (1525 Binary HPLC Pump; Waters). Retention times of NE, epinephrine and dopamine were about 7, 9, and 11 minutes, respectively. Electrochemical detection was performed using model 2464 ECD [Waters; in situ Ag/ AgCl (ISAAC; [Cl–]=0.1 M)].
The levels of GnRH and kisspeptin were measured by specific enzyme-linked immunosorbent assay (ELISA) following supplier’s protocols (MyBioSource, San Diego, CA, USA). Briefly, standard and samples (50 μL) were added to each well and then add the detection reagent A solution (50 μL) immediately. The plate was incubated for 1 hour at 37°C, then the liquid was aspirated and washed 3 times with the wash solution (300 μL). After last washing, the remaining liquid was removed completely. by aspirating or decanting and the detection reagent B solution (50 μL) was added to each well. The plate was incubated for 1 hour at 37°C. After incubation, the plate was washed 5 times as conducted previous washing step. The substrate solution (90 μL) was added to each well and the plate was incubated for 25 minutes at 37°C. When the liquid turned blue with the addition of the substrate solution, the stop solution (90 μL) was added to each well and mixed by tapping the side of the plate. The plate was measured at 450 nM using microplate reader immediately. The detection range of the GnRH ELISA kit was 12.35–1,000 pg/mL and the kisspeptin ELISA kit was 2–700 pg/mL, respectively.
All experiments were performed at least three times. Values were expressed as mean±SE Data were analyzed using Student’s t-test as indicated. p<0.05 was considered statistically significant. Calculations were performed using Graphpad Software Prism version 6 (San Diego, CA, USA).
RESULTS AND DISCUSSION
The norepinephrine (NE) levels in media from NP-treated male and female hypothalamus were not significantly changed across the entire treatment concentration range (from 1 nM to 1 μM), except the level of male 10 nM-treated group which was significantly lower than the levels of control group (p<0.05). The epinephrine (E) levels from NP-treated female hypothalamus were significantly increased in 100 pM- and 1 nM-trated group (p<0.05). However, the E levels from NP-treated male hypothalamus were significantly decreased in 100 pM- and 10 nM-treated group (p<0.05). To our knowledge, this is first report that NP exposure alters the hypothalamic catecholamine secretion.
It is essentially a specialized group of neurons within the adrenal gland that releases catecholamines directly into the bloodstream, rather than communicating across a synapse like typical ganglion neurons, and these specialized cells called chromaffin cells, which are derived from the same embryonic tissue as sympathetic ganglion neurons (Bechmann et al., 2021). Previously we demonstrated that the rat adrenal glands could exert altered NE secretion in response to NP exposure from short-term incubation (Kim et al., 2023).
On the other hand, catecholamine-containing neurons are found throughout rat hypothalamus, with specific distributions in nuclei such are paraventricular nucleus (PVN) and supraoptic nucleus (SON) (Ruggiero et al., 1984). Catecholamines are known to be major neurotransmitters in the hypothalamus, playing a key role in various physiological processes including stress response, feeding behavior, and regulation of hormone secretion (Nagatsu, 2007). In this regard, we thought that one could verify EDC activity of candidate molecule(s) by applying the rat hypothalamus to the short-term culture and examining its catecholamine secretory ability. The present study demonstrates that our assumption is appropriate so NP acts as an EDC in the hypothalamus, altering E secretion during short-term incubation period (Fig. 1B and D).
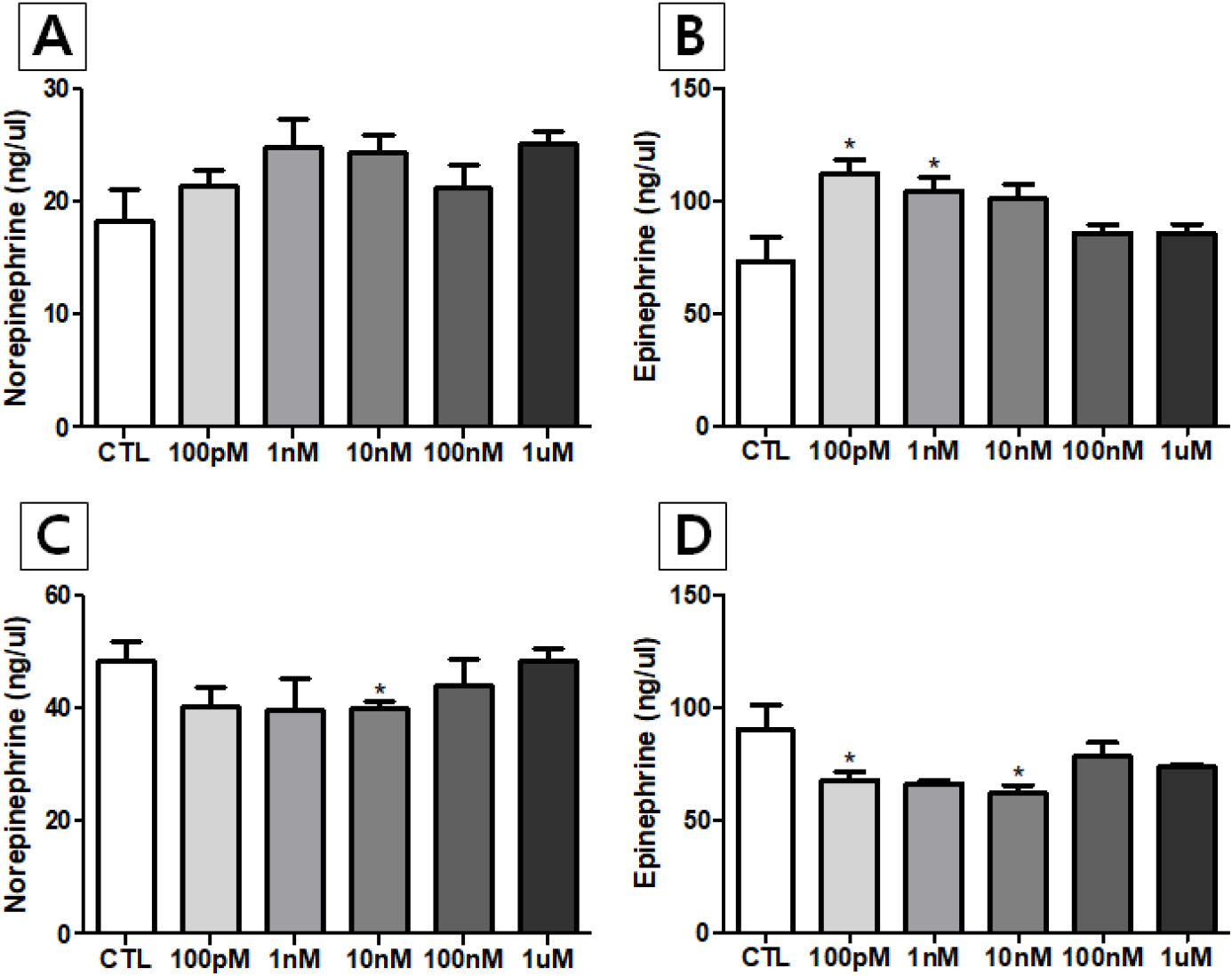
Interestingly, NE secretion of hypothalamus from both sexes in response to NP exposure was not significantly different from the control group except in one experimental group (10 nM group in male). In the previous study, NE secretions of adrenal from female rats in response to NP exposure through 1 nM to 1 μM treatments were significantly higher than the control group, while significant higher levels were found in 100 nM and 1 μM groups in male rats (Kim et al., 2023). SON of the hypothalamus displays sexual dimorphism, which is dependent on body weight, and is relevant to the role of catecholamines in regulating water balance (Madeira et al., 1993). We believed that further studies are needed to determine whether there are sexual dimorphism and/or tissue specificities in EDC action on CA secretion. It will be intriguing that the changes in enzyme activities of enzyme(s) such as phenylethanolamine N-methyltransferase (PNMT), that converts NE to E.
The GnRH levels in media from NP-treated male and female hypothalamus showed an increasing trend, especially significant in male 10 nM- and 100 nM-treated groups (p<0.001) and female 1 nM-, 100 nM- and 1 μM-trated groups (p<0.05, p<0.01, and p<0.05, respectively). Also, the kisspeptin levels in incubated media of both sexes showed a strong increasing trend, especially significant in male 1 nM- and 10 nM-treated groups (p<0.05) and all of female groups except 10 nM-treated group (100 PM treated group, p<0.01; 1 nM-, 100 nM- and 1 μM-treated groups, p<0.001). As shown in catecholamine secretion from adrenals (Kim et al., 2023), this finding is first demonstration that NP exposure alter the rat hypothalamic GnRH and kisspeptin secretions in vitro (Fig. 2).
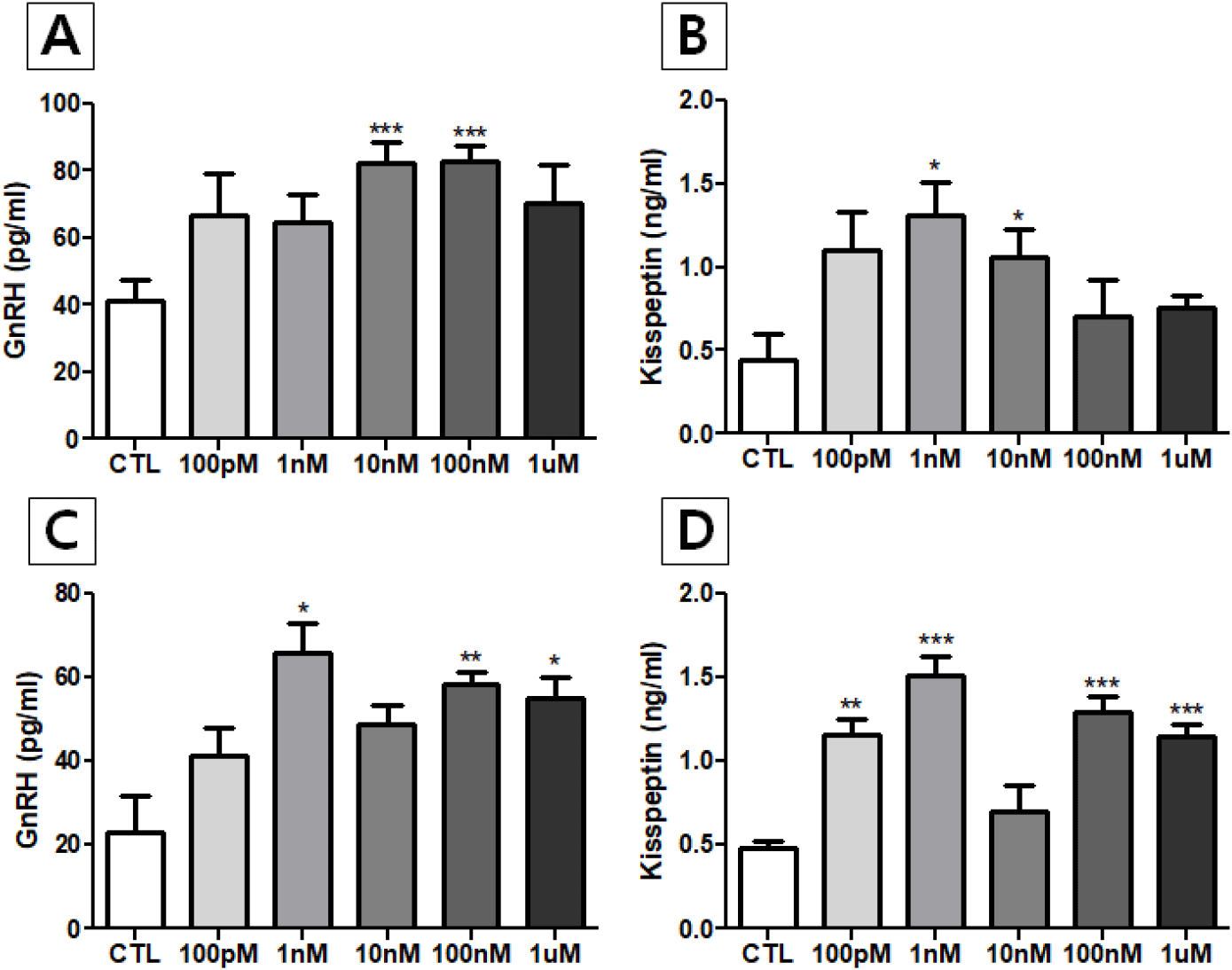
It is now well-known that EDCs can interfere with the hypothalamus, a crucial brain region that regulates hormone production and release, impacting the entire endocrine system. Exposure to EDCs can lead to various health issues, including reproductive problems, metabolic disorders, and neurological dysfunction (Plunk & Richards, 2020; Patisaul, 2021). EDCs mainly affect neuroendocrine hypothalamic function in following ways; (a) Disrupting hormone signals: the hypothalamus controls the pituitary gland, which in turn regulates other endocrine glands. EDCs can mimic, block, or alter the actions of hormones like gonadotropin-releasing hormone (GnRH), which is vital for reproduction (Gore, 2010; Lopez-Rodriguez et al., 2021). There is an evidence that BPA directly decreases GnRH neuronal activity via altered noncanonical intracellular signaling pathway (Klenke et al., 2016), (b) Impact on specific hypothalamic nuclei: There is growing evidence that exposure to EDCs such as BPA, some phthalates, PCBs, polybrominated diphenyl ethers (PBDEs) and biphenyls (PBBs), dichlorodiphenyltrichloroethane (DDT), tributyltin (TBT), and atrazine, are associated with HP axis abnormalities (Graceli et al., 2020). The EDCs can affect different hypothalamic nuclei differently, leading to diverse and sometimes opposing effects. EDCs can affect gene expression specifically in neurons of hypothalamic nuclei, potentially leading to long-term changes in brain development and function (Reilly et al., 2022), (c) Causing neuroinflammation: Some EDCs can induce inflammation in the hypothalamus, potentially disrupting normal neuronal function and hormone signaling (Stathori et al., 2024). Maternal NP exposure induced the proliferation of glial cells, activated microglia and reduced myelin protein expression in oligodendrocytes, resulting negative effects on the brain development in adult male offspring mice (Lee et al., 2024).
Recent studies in rodents have shown that exposure to EDCs can alter hypothalamic circuits involved in appetite, metabolism, reproduction, and other vital functions. These disruptions can lead to various health issues, including reproductive problems, metabolism (including appetite, feeding, and obesity), and developmental delays (Desai et al., 2018; Rezg et al., 2018; Marraudino et al., 2021a,b; Puche-Juarez et al., 2023; Pan et al., 2024; Stathori et al., 2024). Therefore, investigating the effects and action mechanisms of EDC exposure on the hypothalamus is considered very timely from a health and well-being perspective. To understand the precise action mechanism of EDCs on HP axis or hypothalamic level, direct treatment on hypothalamus with EDC could be highly crucial, because the widely used methods such are oral gavage and injections (for example, sc, ip, or iv route) could bring the broad systemic effects. In this regard, organ culture as used in the present study or intracerebroventricular (ICV) injection will guarantee the direct action on hypothalamus, and exclude the indirect effect(s) of extrahypothalamic input.
In conclusion, we developed rat hypothalamus organ incubation system with simple serum-free KRB media for short-term period, and found this method was suitable for detection of catecholamines and neuroendocrine hypothalamic peptides, therefore EDC activity of NP could be easily verified. Treatment with NP altered secretory pattern of E from hypothalamus of both sexes. Interestingly, NP treatment had a stimulatory effect on the female hypothalamus while had an inhibitory effect on the male hypothalamus, showing sexual dimorphism. In addition, NP treatment shown stimulatory effects on both GnRH and kisspeptin secretions from hypothalamus of both sexes, suggesting possible relationship between NP exposure and reproductive phenomena and related disorders.
