INTRODUCTION
Ultraviolet (UV) filters are used as an important component of sunscreens, lotions, shampoos, hairsprays, and other products used to protect the skin from the harmful effects of UV ray (Balmer et al., 2005; Fent et al., 2010; Kim & Choi, 2014). To date, 26 types of UV filters are allowed as cosmetic products according to the European Union (EU) (EU, 2021). Among UV filters, Oxybenzone (Benzophenone-3; BP-3) can block the broadband spectrum, protecting the skin from all the harmful effects of UVA and UVB and thus, is widely used in various personal care products (PCPs) as well as sunscreens to protect human skin and hair from UV damages (Fent et al., 2010; Kim & Choi, 2014).
Indeed, BP-3 is used as an active ingredient in up to 5%–6% of sunscreen products from Korea, Japan, the United States, and Europe (European Commission, 1983; SJP, 1985; KFDA, 2012; US FDA, 2013). BP-3 is also used as food spoilage prevention and deterioration ingredient in food and is approved by the United States Food and Drug Administration (FDA or US FDA) as an indirect food additive (US FDA, 2013). Despite the benefits of BP-3 applications, BP-3 is also associated with endocrine-disrupting potential, such as estrogenic or anti-androgenic effects, and there is growing concern about the environmental release of UV filters containing BP-3 (Schlumpf et al., 2001; Kunz & Fent, 2006; Molins-Delgado et al., 2016; O’Malley et al., 2021). Furthermore, BP-3 has been detected in all areas of fresh and salt water, and has even been reported to accumulate in the bodies of fish and in humans (Poiger et al., 2004; Fent et al., 2010; Kameda et al., 2011). For example, BP-3 can be released indirectly into the aquatic environment via domestic wastewater, with detections reported up to 4.9 μg/L (Leal et al., 2010). BP-3 has also been detected in lakes and streams in Switzerland and Japan at concentrations up to 125 ng/L (Poiger et al., 2004; Fent et al., 2010; Kameda et al., 2011). Recent studies have reported that oxybenzone (BP-3) is frequently detected in marine environments and coastal waters (Tsui et al., 2014; O’Malley et al., 2021). For example, BP-3 concentrations of up to 27.88 μg/L have been observed in coastal waters of Hawaii (Tsui et al., 2014), and in the U.S. Virgin Islands, concentration levels from 74 to 1,400 μg/L have been reported in wastewater effluent from urbanized regions (O’Malley et al., 2021). To reflect both environmentally relevant exposures and possible worst-case scenarios, the present study employed a concentration range of 0, 10, 100, and 1,000 μg/L BP-3. The lower concentrations (10 and 100 μg/L) correspond to the upper levels reported in natural marine and coastal waters, while the highest concentration (1,000 μg/L) represents an extreme scenario that may occur in highly contaminated or enclosed environments. This approach enables a comprehensive assessment of BP-3’s potential effects on estuarine organisms under both typical and high-exposure conditions.
In addition, previous studies have reported that BP-3 accumulates up to 151 ng/g in fish (Fent et al., 2010). Moreover, several studies have reported that BP-3 has been detected in human urine samples (Calafat et al., 2008; Kim & Choi, 2014). Also, some UV filters such as BP-3, 4-Methylbenzylidene camphor (4-MBC), and ethylhexyl methoxycinnamate (EHMC) have been reported to cause embryotoxicity and oxidative stress. For example, negative effects on the development of zebrafish embryos were observed by exposure to BP-3 (Jang et al., 2016). Furthermore, it has been reported that BP-3 may interfere with endocrine system function in fish, where alteration in zebrafish sex ratio and gonadal development was observed at a feminizing Lowest Observed Effect Concentration (LOEC) of 388 mg/L (Schreurs et al., 2002; Kunz & Fent, 2006; Blüthgen et al., 2012; Kim et al., 2014; Kinnberg et al., 2015; Zhang et al., 2017). However, despite these negative effects of BP-3, little is known about the effects of BP-3 on seafood. In this context, the effects of BP-3 on juvenile life history may be greater than those on adults, and may affect hatchability, survival, and development even in the short term. Therefore, it is important to study the effects of BP-3 exposure on the early life history of marine fishes.
The Shimofuri goby (Tridentiger bifasciatus) is a freshwater and estuarine goby that is a fish of the goby family Gobiidae in the order Perciformes (Perciformes), distributed mainly in Asia, including Korea, Japan, China, and the Taiwan region of China (Meng et al., 1994; Matern, 2001). Although not commercially important, the goby family of fishes is ecologically significant due to their adaptability to environments with substantial physical changes and their role as energy transfer agents in coastal food webs (Qin et al., 2020). In particular, T. bifasciatus is a representative species of brackish water habitats, exhibiting a high tolerance to fluctuating salinity and environmental conditions, and playing an important ecological role in estuarine and coastal ecosystems (Matern, 2001). Its wide geographic distribution overlaps with areas exposed to various anthropogenic stressors, such as urban runoff and chemical pollution, making it a suitable indicator species for monitoring environmental changes in estuarine and coastal ecosystems (Whitfield et al., 2012; Qin et al., 2020). Furthermore, T. bifasciatus plays an important role in the food web by consuming benthic invertebrates and serving as prey for higher trophic level organisms (Whitfield et al., 2012). As an environmental indicator species, it has the advantage of small individual size and a shorter life history than other seamount species (Iwata, 2005; Kwak et al., 2010; Qin et al., 2020). Its adaptability to both freshwater and brackish environments, ecological importance in energy transfer, and sensitivity to environmental stress make the T. bifasciatus an appropriate experimental organism for ecotoxicological studies. Therefore, in this study, we investigated the effects of BP-3 on malformations and hatchability during embryogenesis, and on changes in cortisol levels, which are known to play an important role in embryogenesis and growth during fertilization and immediately after hatching, using Shimofuri goby (T. bifasciatus) embryos.
MATERIALS AND METHODS
Oxybenzone (2-Hydroxy-4-methoxybenzophenone, BP-3, CAS number: 131-57-7) used in these experiments was purchased from Sigma-Aldrich (Saint Louise, Mo, USA), dissolved in ethanol at a concentration of 1 mg/mL, and stored at −20°C. Reagents for cortisol assay were purchased from Sigma, and antibodies were from Cosmo-bio (Tokyo, Japan). Radiolabeled hormone was [1,2,6,7-H3]-cortisol purchased from Amersham Life Science (England).
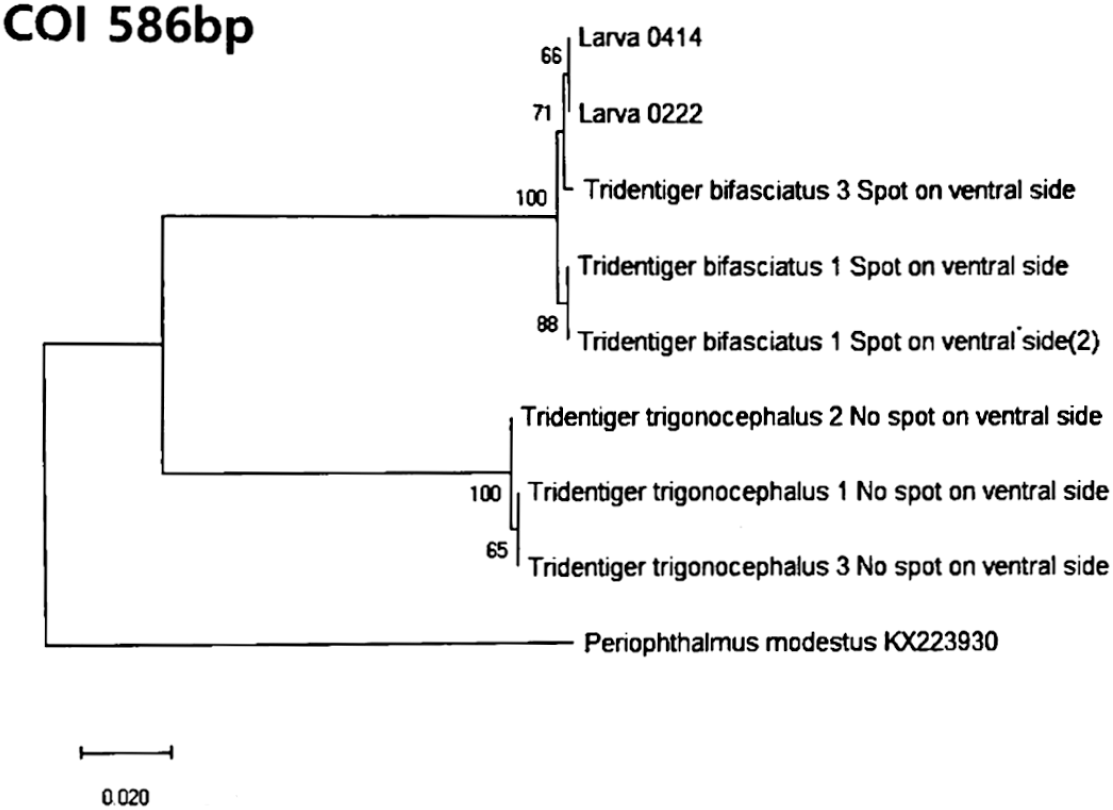
Shimofuri goby (T. bifasciatus) was collected using a float net in October 2020 at Sindeok Beach in Yeosu (Fig. 1). They served as broodstock; artificial fertilization and spawning were performed in the laboratory to obtain larvae, including those referred to as Larva0414 and Larva0222. Thus, Larva0414 and Larva0222 are progeny derived from the adults shown in Fig. 1 and were not collected from the wild. To confirm species identification of goby larvae, both morphological and molecular analyses were conducted. Morphologically, we performed the species identification of the T. trigonocephalus, based on the distribution of white spots on the head and a white stripe in the center of the caudal fin. And for molecular identification, the cytochrome oxidase subunit I (CO1) gene was amplified using genomic DNA extracted from fin tissue and whole larvae using the DNeasy Blood & Tissue Kit (Qiagen, Germany) (Fig. 2). Periophthalmus modestus (GenBank Accession No. KX223930) was included as an outgroup species in the COI phylogenetic analysis for tree rooting and comparison (Fig. 2). They were acclimatized for 2 weeks in laboratory aquaria after a 1-hour bath with oxytetracycline (100 ppm) before being housed in indoor aquaria. Artificial seawater was used throughout the experiment, including during egg exposure and water replacement, to ensure consistency and minimize variability caused by changes in water composition. The rearing conditions during the larval period were controlled at water temperature of 20.0±0.5°C, photoperiod of 12L:2D (Light:Dark), and feeding regimen of compound diet twice daily.
After a 2-week acclimatization period, spawning was induced by adjusting the photoperiod in 2-week increments to 12L:12D for weeks 0 to 2, 13L:11D for weeks 2 to 4, and 14L:10D for weeks 4 to 6, with a water temperature of 21.0±0.5°C and a photoperiod of 14L:10D. The spawners were maintained at 21.0±0.5°C and a light intensity of 14L:10D. The PVC pipe (15 cm length×5 cm diameter) was isolated from the tank when we observed occasional spawning on PVC pipe, to confirm that the female had completed spawning, or when we found the male guarding the eggs after spawning, and the fertilized eggs attached to the pipe were removed. To reduce the delay between spawning and egg collection, spawning activity and the condition of the PVC pipe were monitored frequently throughout the day at short intervals. Exposure experiments were performed by transferring the fertilized eggs (n=30 per group) removed from the pipe to 6-well plates containing 21.0±0.5°C, 30 Practical Salinity Units artificial seawater (Instant Ocean® Sea Salt, Spectrum Brands, Blacksburg, VA, USA) with BP-3 dissolved at concentrations of 0, 10, 100 and 1,000 μg/L. All BP-3 stock solutions were prepared in ethanol and diluted to a final solvent concentration of 0.01% (v/v) in each treatment. A separate solvent control group was not included because previous studies have demonstrated that 0.01% ethanol does not induce developmental toxicity in teleost embryos (Jang et al., 2016; Torres et al., 2016). Therefore, the 0 μg/L group served as the blank control without ethanol. The experiment was terminated immediately after hatching and was repeated three times. The experiments focused on embryonic development of the T. bifasciatus, and live embryos were observed every 12 hours using a light microscope equipped with an image acquisition device.
Survival rates are recorded every 12 hours, and dead individuals were removed upon identification. Dead individuals were selected by the presence or absence of a heartbeat 84 hours post fertilization, when the heart was fully formed, and the survival rate (SR, %) was calculated as following:
Survival number (SN) indicates the number of surviving individuals. SNtx and SNt0 represent the number of oocytes alive at x hours post-exposure (×HPE) and at the start of the experiment (0 HPE), respectively.
To estimate the lethal concentration (LC50) value, mortality data at each BP-3 concentration were plotted and analyzed using probit analysis with SPSS Statistics software (IBM, Armonk, NY, USA). The LC50-96h was defined as the concentration causing 50% mortality at 96 hours.
The hatching rate (HR, %) at the end of the exposure experiment (108 HPE) was calculated as following:
HNt108h represents the number of individuals hatched and alive at 108 hours after exposure.
HR was measured by counting the number of heartbeats in a 20-second period in those fertilized eggs that were exposed to BP-3 during 60 hours. All measures (SR, HS, and HR) are calculated based on the average of the triplicates.
Cortisol contents in fertilized eggs exposed to BP-3 during early development were analyzed by radioimmunoassay (RIA). The cortisol content was measured by specific RIA as described by Seth & Brown (1978). Fertilized eggs for RIA analysis were placed in a balanced salt solution (BSS, pH: 7.7, osmotic concentration: 360 mOsm/kg) at approximately 100 eggs per replicate and stored at −80°C until analysis. Sampling was done at 0, 24, and 96 h of exposure to BP-3 (0 [untreated control], 10, 100, and 1,000 μg/L) (n=3). Sampled eggs were weighed to the nearest 0.1 mg using a microbalance, crushed using a homogenizer, and the supernatant was obtained after centrifugation at 16,110×g (13,000 rpm, rotor radius 9.5 cm) for 5 min at 4°C to determine the cortisol concentration using a liquid scintillation counter (Packard Tri-Carb® 2100TR, Canto, MA, USA). The extraction solvent was ethyl acetate: cyclohexane (1:1, v/v) for two repeated extractions, and the measured cortisol concentrations ranged from 15 pg/mL to 3,840 pg/mL.
Hatching rate, survival rate, heart rate, and cortisol levels of T. bifasciatus embryos were expressed as mean±SD. Prior to statistical comparison, data were tested for normality using the Shapiro–Wilk test and for homogeneity of variances using Levene’s test to ensure the assumptions for parametric analysis were met. One-way analysis of variance (ANOVA) was conducted using IBM SPSS Statistics (version 21.0), followed by Tukey’s HSD post hoc test to assess differences among treatment groups. Statistical significance was accepted at p<0.05.
RESULTS
In this study, the toxic effects of BP-3 on embryonic development of T. bifasciatus, were observed. (Fig. 3). After 36 hours of exposure, the tails had not yet separated from the yolk after exposed to 100 and 1,000 μg/L BP-3. After 60 hours of exposure, heart formation was completed and active heartbeats were observed in individuals in the control and 10 μg/L BP-3. In contrast, irregular or weak heartbeats and maldeveloped cardiac structures were observed in the 100 and 1,000 μg/L BP-3. At 72 hours after exposure, eye pigmentation was completed in the control group, while it remained incomplete in embryos from all BP-3 treatment groups (10, 100, and 1,000 μg/L). After 48 and 60 hours of exposure, all individuals in the control group had elongated tails, with the tail positioned at the tip of the head, whereas individuals in the all BP-3 treated group failed to elongate or showed signs of bent tails.
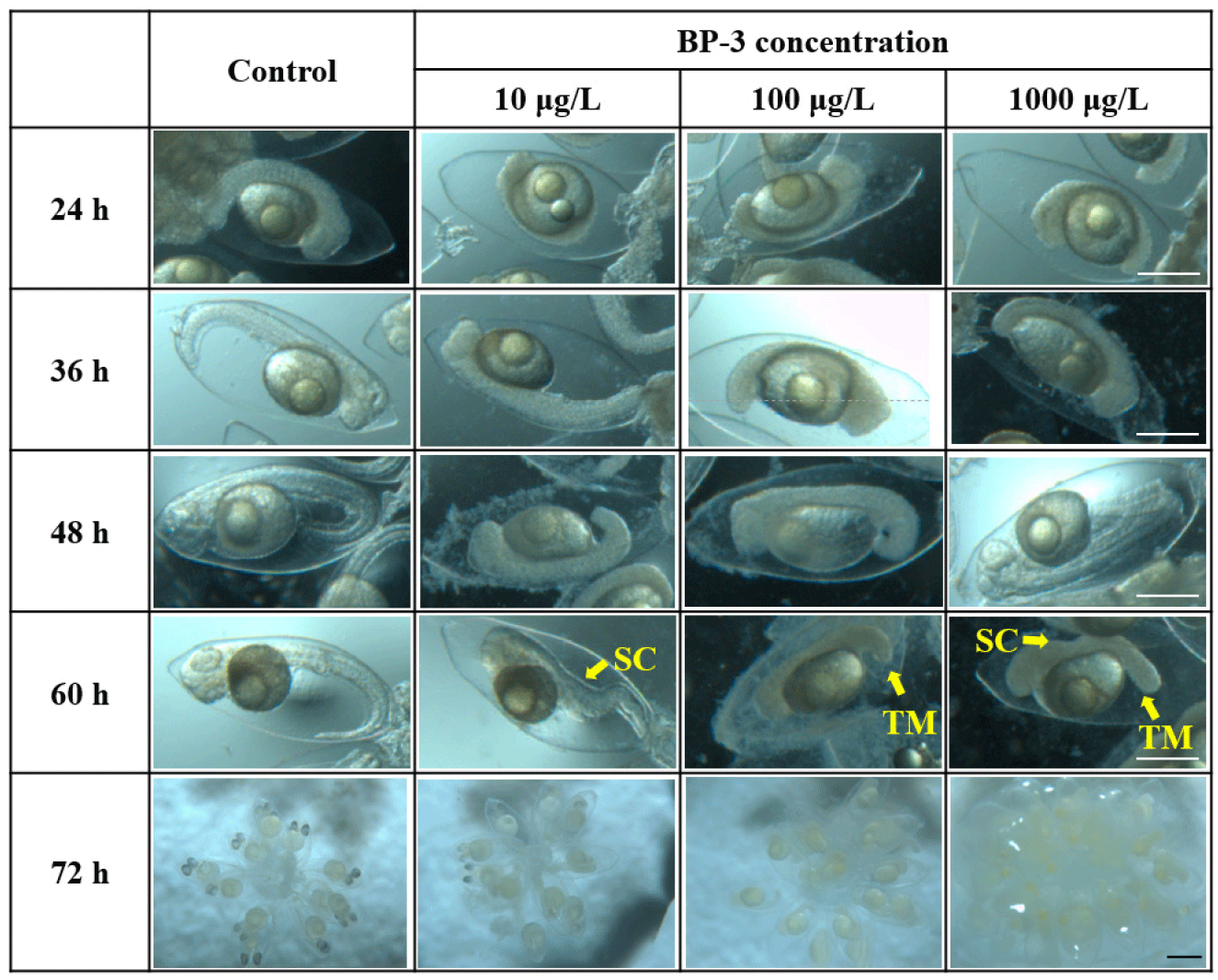
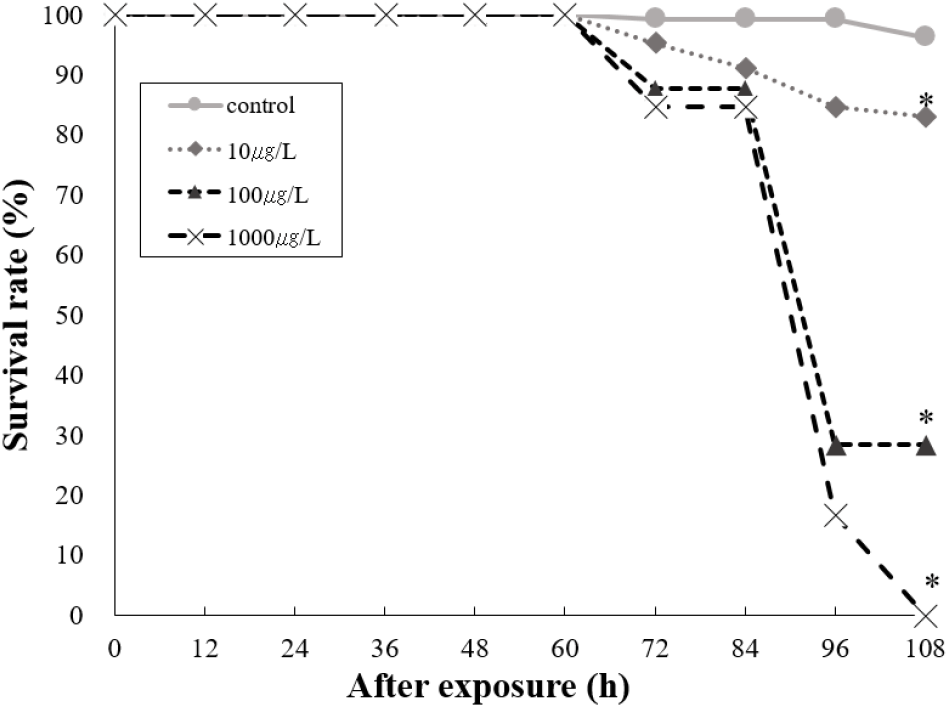
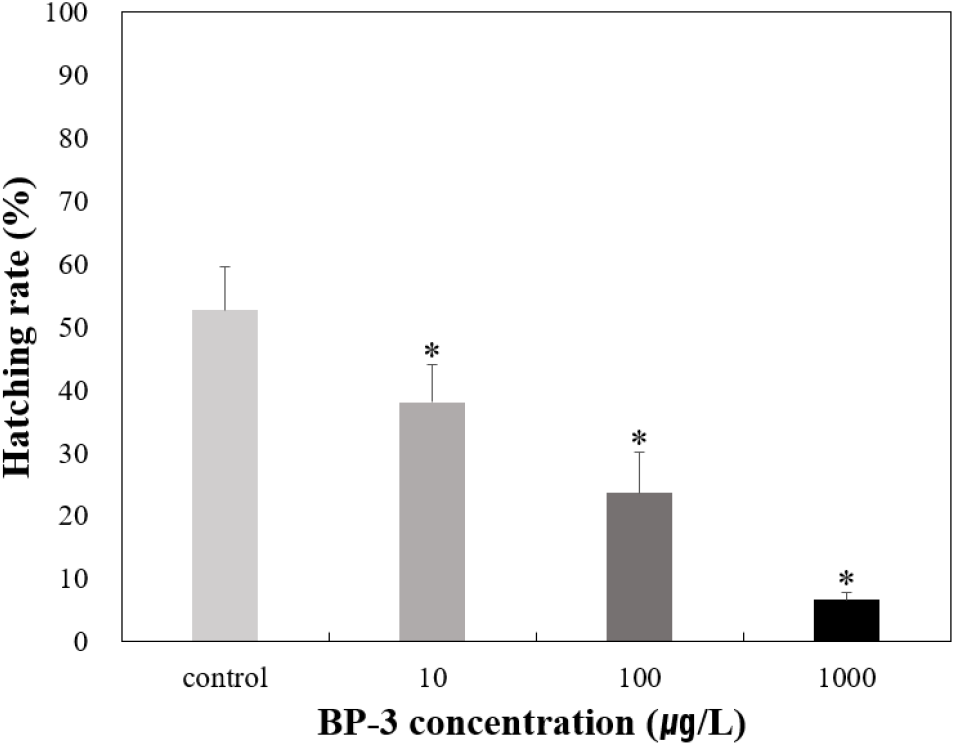
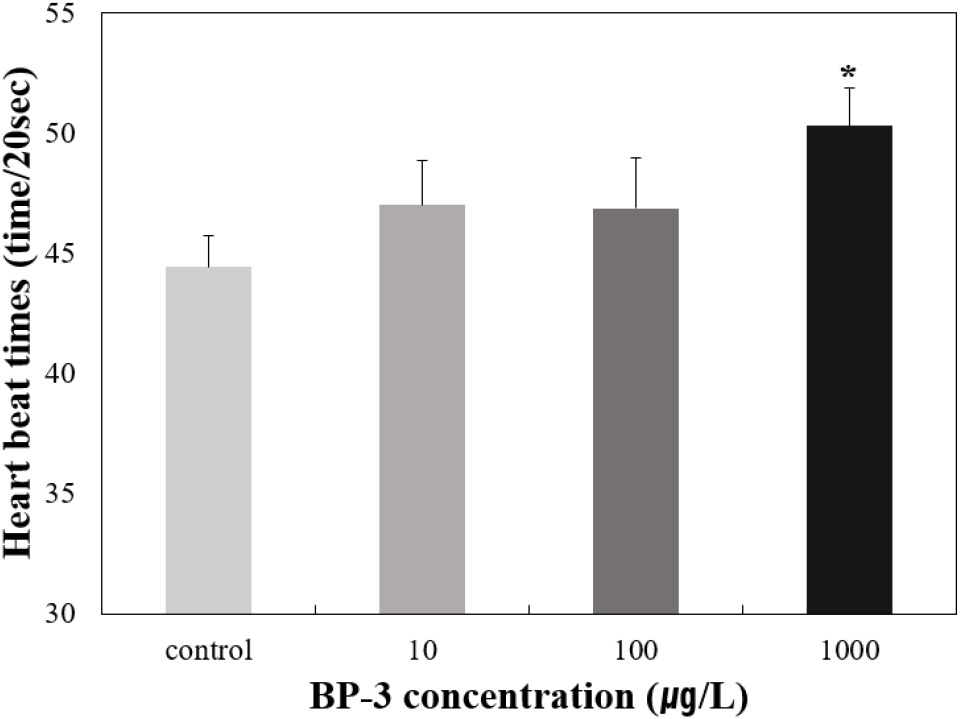
To determine the effects on survival and HR of T. bifasciatus embryos, early fertilized eggs were exposed to BP-3 (0 [untreated control], 10, 100, and 1,000 μg/L). Individuals with no heartbeat or unformed hearts were determined to be dead after 72 hours of exposure under microscopic observation. Survival rate of T. bifasciatus embryos was greater than 90% in the control group, whereas all BP-3 treated groups showed rapid mortality between 84 and 96 hours of exposure (immediately after hatching) (Fig. 4). Survival rates at 84 and 96 hours were 99.3% and 99.3% in the control group, 91.1% and 84.7% at 10 μg/L, 87.6% and 28.5% at 100 μg/L, and 84.7% and 16.7% at 1,000 μg/L, respectively. These results indicate a dose-dependent decrease in survival rate during the critical post-hatching period (p<0.05). Hatching rate of T. bifasciatus embryos decreased in BP-3 concentration-dependent manner (Fig. 5). The HRs (mean±SD) were 52.7±6.8% in the control group, 38.0±6.1% at 10 μg/L BP-3, 23.7±6.4% at 100 μg/L, and 6.7±1.2% at 1,000 μg/L. All BP-3-treated experimental groups showed a significant difference compared to the control group (p<0.05). Also, heart rate was increased in response to BP-3 (Fig. 6). In particular, those individuals with a complete heart development showed that the control groups tend to have significantly less heart rate (44.4±1.4 beats per 20 seconds) compared to those exposed to BP-3 concentration of 1,000 μg/L for 60 h (50.3±1.7 beats per 20 seconds) (p<0.05) (Fig. 6).
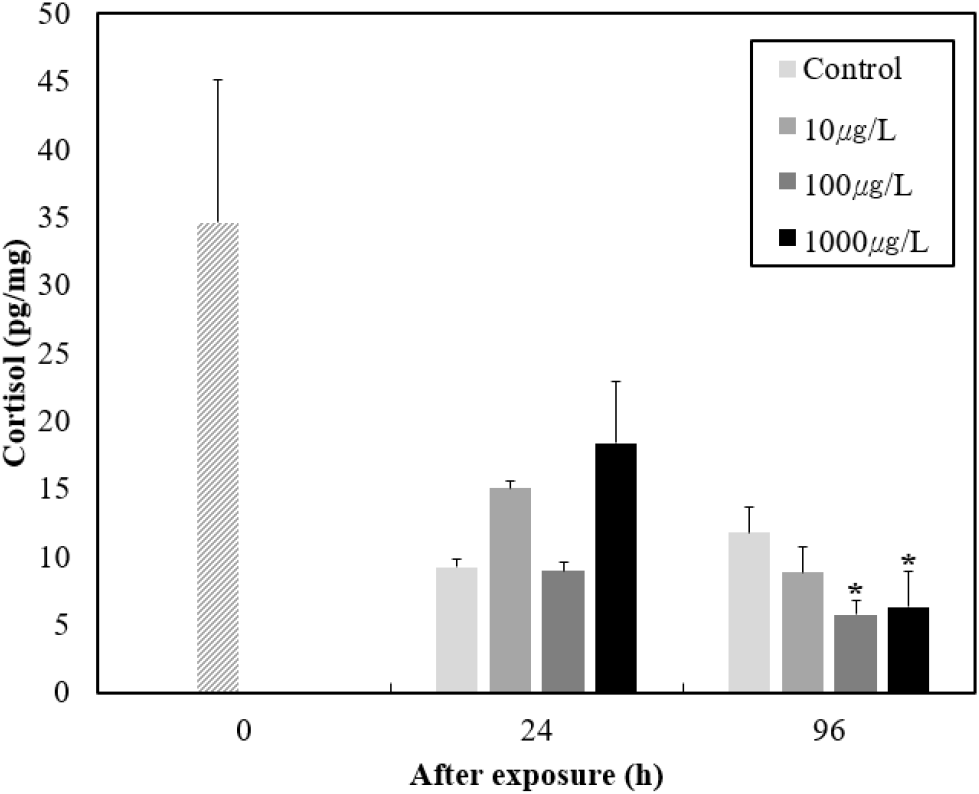
To explore endocrine involvement, we measured the cortisol levels in response to BP-3 (Fig. 7). Immediately after exposure, cortisol levels were 34.6±10.5 pg/mL, but cortisol levels decreased as the developmental process progressed. Particularly, very low cortisol levels were seen in the BP-3 100 μg/L group at 96 hours post-exposure compared to the control group.
DISCUSSION
In a marine environment, BP-3 that is a common UV filter in sunscreens can be significantly harmful to marine organisms and ecosystems, acting as an endocrine disruptor that can interferes with hormonal systems, leading to developmental retardation and reduced reproduction. However, toxic effects of BP-3 on marine organisms are still poorly understood. Therefore, it is necessary to improve our understanding of how BP-3 affect the physiological response, particularly on embryo development, in marine organisms.
In this study, abnormalities in embryonic development of T. bifasciatus were observed in response to BP-3. These morphological impairments are consistent with previous findings in marine fish. In a previous study, malformations in zebrafish embryonic development were not observed in low exposure concentrations (≤300 μg/L) of BP-3 (Wang et al., 2023). However, during embryonic development, spine and tail malformation were observed in the rock bream (Oplegnathus fasciatus) exposed to BP-3 (Song & Baek, 2022). Similarly, Wang et al. (2021) reported that the average malformation rates was increased in BP-3 concentration dependent manners, suggesting the difference in species-specific sensitive to BP-3 and BP-3 can induce adverse effect on embryonic development in T. bifasciatus.
In this study, decrease in survival rate was observed in response to BP-3. Also, lethal concentration (LC50-96 hours) value of BP-3 was 493 μg/L in T. bifasciatus embryos. This is considerably lower than values reported in other species. For example, the LC50-96 h for BP-3 in Japanese Medaka, Oryzias latipes, is 3.8 mg/L and 15.93 mg/L in zebrafish larvae after 96 h postfertilization of exposure (Balázs et al., 2016; ECHA, 2021). Also, in zebrafish embryos, LC50-24 hours were 4.19±3.60 mg/L (Ortiz-Román et al., 2024). Considering this, we have found that T. bifasciatus embryos are highly sensitive to BP-3 compared to other fish species. Although no mortality was observed in some studies using zebrafish embryos (Cahova et al., 2021; Lucas et al., 2021), delayed hatching, impaired ovarian development, and embryonic malformations were reported under BP-3 exposure (Wang et al., 2023; Ortiz-Román et al., 2024). These findings support a mechanistic link between BP-3 exposure and embryonic maldevelopment in T. bifasciatus and suggest that BP-3 can induce embryonic malformations, leading to a significant effect on the survival of embryonic-larval stages of T. bifasciatus.
In this study, decrease in HR of T. bifasciatus embryos was observed in response to BP-3. In general, HR has been widely used as an important endpoint of toxic effects on early development (OECD, 2013; Samaee et al., 2015; Torres et al., 2016; Cao et al., 2019). In previous studies, the number of eggs laid and HR were monitored after exposing adult trout, O. latipes, to 10, 100, and 1,000 μg/L BP-3 for 21 days, and found that HR was significantly reduced at 1,000 μg/L of BP-3 (Coronado et al., 2008). A significant reduction in HR was also observed when zebrafish embryos were exposed to BP-3 (Balázs et al., 2016). In O. fasciatus embryos, decrease in HR was observed in the BP-3 exposure (Song & Baek, 2022). In this regard, this delayed hatching and reduced HR is possibly due to spinal curvature or inhibition of hatching enzymes, which prevent the embryo from hatching from the yolk sac (Sun & Liu, 2017). In addition, embryos exposed to BP-3 may fail to hatch because the tail is deformed, which impedes embryo movement (Coronado et al. 2008). Similarly, in this study, BP-3 caused tail deformation in T. bifasciatus embryos (Fig. 3). Indeed, in O. fasciatus embryos exposed to BP-3, tail and spine malformations were observed, which were positively correlated with a decrease in hatching (Song & Baek, 2022). In addition, Incardona et al. (2004) reported that cardiac dysfunction can lead to malformations such as dorsal and caudal curvature, suggesting that BP-3 can induce tail and spine malformations associated with cardiac dysfunction, resulting in inhibition of hatching success of T. bifasciatus embryos. Taken together, our results suggest that BP-3 can have adverse effects on the survival and hatching success of T. bifasciatus embryos.
In this study, a trend toward increased heart rate was observed with BP-3 exposure. Generally, heart rate is a useful tool in early developmental toxicity testing (Suvarchala & Philip, 2016). In previous studies, some UV filters have been shown to be heart rate mediated embryotoxic. For example, Jang et al. (2016) observed a decrease in heart rate in wild-type zebrafish after exposure to Ethylhexyl methoxycinnamate (OMC), one of the UV filters, and reported that it was dose- and time-dependent manners. In addition, Torres et al. (2016) reported that exposure to 4-MBC lead to a decrease in heart rate of zebrafish embryos. Indeed, deteriorated cardiac contractility was observed in BP-3 exposed to zebrafish embryo (Jang et al., 2016). However, 2-ethylhexyl salicylate significantly increased the cardiac frequency in zebrafish embryos (Lucas et al., 2022). Also, embryo-larval stages of zebrafish, heartbeat was increased after exposure to the bis-Ethylhexyloxyphenol methoxyphenyl triazine (Lucas et al., 2021), indicating difference in species-specific toxicity to UV filter difference. These findings highlight species- and compound-specific cardiotoxicity patterns. Overall, our result suggests that BP-3 can induce adverse effects on heart rate in the T. bifasciatus embryos. In this regard, Li et al. (2018) confirmed that malformations occurring in the heart and pericardium can affect heart function, causing irregular heartbeats and impaired blood flow. In addition, in this process, previous study reported that cortisol likely affects the functionalities of heart and development in zebrafish embryos (Nesan & Vijayan, 2012). Generally, cortisol, a steroid hormone, is known to be involved in hatching and growth during early embryogenesis in fish, and also is an endocrine signaling molecule in all vertebrates and plays an important role in many organ and biological functions including immune function, behavior, stress, metabolism, growth, and organogenesis, including the respiratory, renal, and cardiovascular systems (Wintour, 2006; Simontacchi et al., 2008; Trayer et al., 2013). In fish, maternal cortisol delivered to the oocyte is the sole source of steroids until hatching and is essential for early development, including maturation (Nesan & Vijayan, 2013; Nesan & Vijayan, 2016). Furthermore, cortisol plays an important role in mobilizing energy stores into a form that is readily available to the developing embryos (McCormick & Nechaev, 2002). Increased heart rate in our study may reflect compensatory responses to early-stage cardiac stress, yet the mechanistic basis remains unclear, as gene expression or molecular pathway data were not analyzed.
To further address the knowledge gap, we confirmed the change in cortisol levels in response to BP-3. In general, changes in the cortisol levels can directly affect heart growth as the heart-forming hormone in the embryo (Wintour, 2006). Furthermore, cortisol deficiency during development may impair energy mobilization, resulting in growth plateau phases and structural malformation (Mathiyalagan et al., 1996; Mccormick & Nechaev, 2002). Indeed, this was consistent with our findings. Exposure to BP-3 increased abnormal heart formation and heart rate in T. bifasciatus embryos. In a previous study, in the damselfish (Pomacentrus amboinensis) eggs, embryonic heart formation appeared 1 to 1.5 hours earlier in cortisol-exposed embryos than in controls, and the initial heart rate of cortisol-exposed embryos was observed to be higher than in controls (Mccormick & Nechaev, 2002). In addition, zebrafish embryos exhibit malformations with dilated heart cavities when exposed to cortisol-treated environments, and a rapid increase in heart rate when exposed to cortisol solutions for more than 48 hours. In addition, a previous study reported that elevated cortisol content in embryos increased cardiac malformations, including pericardial edema and ventricular malformations (Nesan & Vijayan, 2012). In this regard, Mccormick & Nechaev (2002) reported that the acceleration of embryonic growth induced by cortisol leads to a decrease in cortisol levels and an increase in energy expenditure, but energy replenishment may not be able to supply the demand, and this leads to alternating periods of growth and growth plateau during the early stages of development due to energy insufficiency, and this discontinuous growth pattern in P. amboinensis embryos resulted in an overall decrease in growth, as cortisol-exposed embryos had a higher relative growth rate for a short period of time compared to controls, but a longer period of growth plateau. Also, a study which conducted exposure study using tilapia larvae (Oreochromis mossambicus) to various concentrations of cortisol for two weeks found that cortisol not only affects growth, but also accelerates yolk absorption and swimming activity in the larvae (Mathiyalagan et al., 1996), suggesting that change in cortisol levels is likely related to embryonic and larval development of fishes. Taken together, BP-3 causes changes in cortisol levels, which lead to adverse effects on survival, HR, heart rate, and deformities of T. bifasciatus embryo.
Benzophenone-3 (BP-3) causes severe morphological abnormalities, cardiac dysfunction, and endocrine disruption during the embryonic development of the Shimofuri goby, T. bifasciatus. This study provides novel evidence that T. bifasciatus embryos are more sensitive to BP-3 than other model fish species, highlighting its utility as a potential ecotoxicological indicator. However, the long-term effects of early-life exposure to BP-3 remain unclear. Future studies should investigate post-hatching growth, reproductive capacity, and chronic low-dose exposure to better reflect environmentally relevant risks. By elucidating both acute and delayed toxicity pathways, these efforts will contribute to a more comprehensive ecological risk assessment of UV filters in marine ecosystems.
